Consequences of the Second Law - summary
| Thermodynamics | |
|---|---|
| Introduction | What is this thing called Thermodynamics??? | Definitions | Thermal Equilibrium and Zeroth Law | Limitations |
| First Law | Work, Heat, Energy, and the First Law | Work, Heat, Energy, and the First Law (simplied) | Derivatives | Derivatives Exercise | Reversibility, Enthalpy, and Heat Capacity |
| Second Law | Things to Think About | Observations and Second Law of Thermodynamics | Alternative Approach - the Clausis Inequality | Consequences of the Second Law | Consequences of the Second Law (simplified) | Carnot Principle - motivation and examples | Equivalence of Second Law Statements* |
| Third Law | Third Law of Thermodynamics | Consequences of Third Law* |
| Development of Thermodynamics | The Thermodynamic Network | Network Exercise | Equations of State (EOS) | EOS Example, Reading Tables, and Numerical Analysis | EOS Exercises | Thermochemistry |
* Optional Section | |
Note: The following is a summary of results from the second law of thermodynamics. More information can be found in the longer section on Consequences of the Second Law
Contents
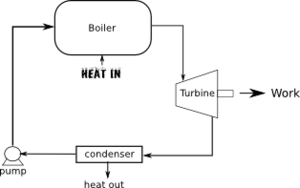
Heat Engines
A Heat Engine is a cyclic process which converts heat into energy. By cyclic we mean that the process begins and ends in the same state. The cycle can either been within a single device or in devices linked together. The two most common types of heat engines are the internal combustion engine in a car and the electric power plant
The second law of thermodynamics says No system can be developed which completely converts heat to work. So the second law should give some insight into heat engines. In doing so we will also learn some things about energy in general.
Since all of the heat entering a heat engine is not converted to work there must be some leftover heat. This is called waste heat.
Efficiency
- Efficiency, η
- The ratio of the net work to the heat input.
[math]\eta=\frac{-W}{Q_{in}}[/math]
Therefore, efficiency is the fraction of heat which is converted to work.
Carnot Cycle
The Carnot cycle is a theoretical heat engine. Its efficiency is
[math]\eta_{Carnot}=\frac{T_H-T_C}{T_H}[/math]
where TH is the temperature of the heat source and TC is the temperature of the heat released. (Remember in thermodynamics all temperatures are absolute)
Note that this is efficiency is based on only the temperatures, not on the internal workings of the cycle. It leads to the Carnot Principle:
The maximum efficiency of a real cycle is the efficiency of a Carnot cycle operating over the same temperatures
The Carnot efficiency is often used a limiting efficiency and to compare with real efficiencies.
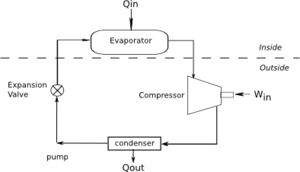
Refrigerators
A refrigerator is a device which removes heat from a colder region to a warm region. Note that an air conditioner is really a type of refrigerator. Figure 2 shows a diagram of a refrigerator.
COP
For a heat engine we are interested in getting the maximum amount of work out. However, for a refrigerator, we are interested in the minimum work we have to put in. Therefore, efficiency is not the best measure. Instead we use the Coefficient of Performance (COP). For a refrigerator the COP is:
[math]COP_R=\frac{Q_{in}}{W}[/math]
Efficiencies
Power Plants
A typical power plant has a Carnot efficiency of about 45%.[1]
Real efficiencies are at most 75% of the Carnot efficiency. Therefore, the overall efficiency is about 30%.
In other words, only about one-third of the energy from burning the fuel is converted to work. The rest is expelled as heat.
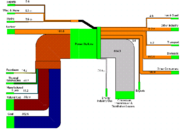
Figure 2 is an electricity flow chart for the United Kingdom in the year 2007. It shows input resources and output uses of electricity, where the width of the bars are proportional to the amount of energy. We can see the effects of efficiency by looking at the conversion losses (the gray bar), which dwarfs the other outputs.
Other Efficiencies
Let us at the efficiencies of some other processes:
- Fuel Cell 60%
- Human Body 25%
- Florescent Light 15%
- Car Engine 10%
- Incandescent Light 5%
The human body is only 25% efficient, which is why a room full of people is warmer than a empty room - all those people are giving off heat.
G. Tyler Miller calls an incandescent light bulb a "heat bulb" because it produces much more heat than light. The difference in efficiencies between types of light bulbs is why compact fluorescent lights are recommended over incandescent lights.
Consequences
- The concept of efficiency is the basis behind the all the concerns with energy issues. But note that the term energy crisis is a misnomer, energy is conserved. Rather it is a crisis in the quality of energy.
- There has been renewed interest in "renewable energies" - technology such as wind energy. Most of these (but not all) belong to what are called direct energy conversions. These do not convert heat to work (and therefore do not run in a cycle) and so are not limited by the Carnot efficiency.
- One practice that can be used with a thermal power plant is cogeneration, which is where the excess heat expelled is used to heat either an industrial process or buildings.
- The heat expelled from a cycle must go somewhere. It is usually captured by water which is then released into a lake or the sea. This creates "thermal pollution". This changes the temperature of the receiving water, which causes both a physical change (e.g. reduction of dissolved oxygen) and a physiological change in fish and other animals.
Life and Order
Life involves taking small molecules and creating larger units such as proteins and starch. These are then further put together to form structures such as skin or muscle. In other words it involves creating order. Hence, it reduces the entropy.
Since life reduces entropy, the entropy of the environment must increase. Hence it releases waste heat.
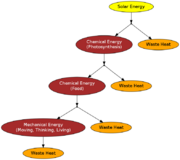
Figure 3 shows how energy is transformed as is flows through a simplified biological system. The ultimate source of all energy in living systems is solar energy[2]. This energy is then converted to chemical energy through photosynthesis. This is then converted to food, which is then converted to mechanical energy for thinking, moving, and doing other functions.
Perpetual Motion Machines
A perpetual motion machine is is literally a machine which is supposed to continue forever. There are two types: A perpetual motion machine of the first type (PMM1) is machine that claims to create, instead of conserving, energy. It violates the first law of thermodynamics. A perpetual motion machine of the second type (PMM2) is a machine that claims to violate the second law of thermodynamics. (There is also a perpetual motion machine of the third type (PMM3) - which are not related to thermodynamics, but rather supposedly has no dissipation of energy).
PMM2 attempt to convert heat completely to work. They produce work solely by cooling of a body. Hence, no heat is released from the machine. In other words, whereas a heat engine has two heat reservoirs (one which is cooled and the other heated), a PMM2 has only one reservoir (the one cooled).
All perpetual motion machines are inconsistent with the laws of thermodynamics and therefore cannot exist.
A good article on perpetual motion machines can be found on Wikipedia
No Hope
Remember this quote whenever you here about perpetual motion machines or another dubious statements:
The second law of thermodynamics holds, I think, the supreme position among laws of nature...If your theory is found to be against the second law of thermodynamics, I can give you no hope.
- Arthur S. Eddington