The Contact Process
Chemical Equilibria|Le Chatelier's Principle|Factors Affecting Chemical equilibria| The Haber Process|The Contact Process|Equilibrium Constants
The Contact Process
The Contact process is the process during which sulphur trioxide (SO3) is formed from sulphur dioxide (SO2).
The Sulphur trioxide which is formed is then used to manufacture sulphuric acid.
Sulphur dioxide can be made by either burning sulphur in excess of air or by roasting of sulphide ores.
The manufacture of sulphur trioxide is a reversible reaction and its enthalpy change is exothermic.
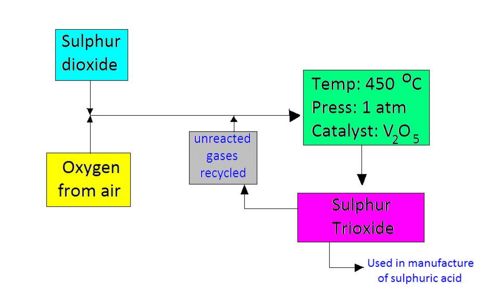
The following shows a flowchart of the reaction scheme:
2SO2(g) + O2(g) <=> 2SO3(g) ∆H = -196 kJ/mol
Conditions required for the reaction:
1. Temperature: 450 oC
2. Pressure : 1 atm
3. Catalyst: Vanadium (V) oxide, V2O5
Explaining the conditions:
Temperature
A temperature of 450 oC is used in this reaction. This is because the reaction is exothermic such that a low temperature would favour the forward reaction and produce more sulphur trioxide. However, a low temperature slows down the rate of the reaction. Thus a compromise temperature of 450 oC is used.
Pressure
A pressure of only 1 atm is used. According to LCP, an increase in pressure would increase the yield of the reaction because there are fewer number of moles of gas on the right hand side. However, a pressure of 1 atm is enough to obtain a very good yield. Thus it would be a waste of money and resources to increase the pressure.
Catalyst
Vanadium (V) Oxide, V2O5 is used as catalyst in this reaction. It increases the rate of the reaction, thus reducing the need for a high temperature.
Manufacture of Sulphuric acid
The sulphur trioxide obtained is then used to manufacture concentrated sulphuric acid.
Step 1:
Burning of sulphur
S + O2 --> SO2
Step 2:
Converting sulphur dioxide into sulphur trioxide
2SO2 + O2 <=> 2SO3
Step 3:
Adding concentrated sulphuric acid to sulphur trioxide to form oily product known as oleum.
SO3 + H2SO4 --> H2S2O7
Step 4:
Adding a calculated amount of water to form sulphuric acid.
H2S2O7 + H2O --> 2H2SO4