Factors Affecting Chemical equilibria
Chemical Equilibria|Le Chatelier's Principle|Factors Affecting Chemical equilibria| The Haber Process|The Contact Process|Equilibrium Constants
Contents
Factors affecting Equilibria
There are 3 main factors that affect the equilibrium of a reaction:
- Concentration of reactants or products
- Temperature
- Pressure (Affects only gaseous reactions)
Concentration of reactants/products
Let us consider the following general reaction:
A + B <=> C + D
Changing the concentration of reactants
*Increasing concentration
If the concentration of A is increased, according to LCP, the system will shift in such a way so as to decrease the concentration of A. It can only do so by shifting forward, to the right. As a result, the concentration of A and B decreases and that of C and D increases.
*Decreasing concentration
If the concentration of B is decreased, according to LCP,the system will shift in such a way so as to cancel the change. It can only do this by shifting backwards, to the left to restore the equilibrium. As a result, the concentations of A and B increases and those of C and D decreases.
Changing concentration of products
*Increasing concentration
If the concentration of C is increased, according to LCP, the system will shift in such a way so as to decrease the concentration of C. It can only do so by shifting backwards, to the left. As a result, the concentration of A and B increases and that of C and D decreases.
*Decreasing concentration
If the concentration of D is decreased, according to LCP,the system will shift in such a way so as to cancel the change. It can only do this by shifting forward, to the left to restore the equilibrium. As a result, the concentations of A and B decreases and those of C and D increases.
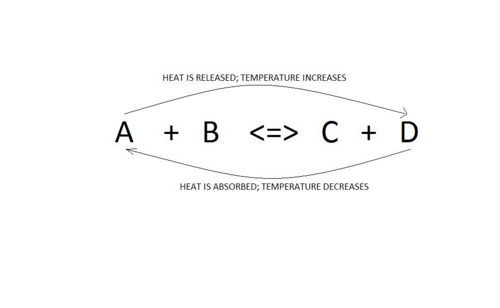
Temperature of system
Changing the temperature
When considering changes in temperature, one must also consider the enthalpy of the reaction.
Let us consider the following general reaction:
A + B <=> C + D ∆H = -ve (Reaction is exothermic)
*Increasing temperature
An increase in temperature will cause the equilibrium to shift backwards.This is because the system will try to get rid of this excess heat and can do so by going to the lft where heat is absorbed. Thus, the amounts of A and B will increase and those of C and D will decrease.
*Decreasing temperature
A decrease in temperature will favour the forward reaction. This is beause the sysem will try to restore the initial temperature and can only do so by moving forward where heat is released. Thus, the amounts of A abd B decreases and those of C and D increases.
Pressure of system
Pressure affects only gaseous reactions, that is reactions which contain gaseous particles.
Let us consider the following general gaseous equation
2A (g) + B(g) <=> C (g) + 3D(g)
The very first thing to do before deciding how the system will shift according to LCP when a chnage in pressure is applied, is top dermine the total number of moles of gases on each side of the equation. This gives an indication of the pressure according to Ideal Gas Equation.
From the above equation, we can deduce that here are 3 moles of gas on the left hand side 2 moles of A and 1 mole of B) and 4 moles of gas on the right hand side(1 mole of C and 3 moles of D).
Thus we conclude that there is a higher pressure on the right hand side as compared to the left hand side.
2A (g) + B(g) <=> C (g) + 3D(g)
3 moles of gas 4 moles of gas
Lower pressure Higher pressure
*Increasing the pressure
An increase in pressure causes the equilibrium to shift backwards. This is because according to LCP, the system will try to decrease the pressure and can only do so by shifting the equilibrium to the side where there is less number of moles that is the left hand side. Thus the amount of A and B increases and that of C and D decreases.
*Decreasing the pressure
A decrease in pressure causes the equilibrium to shift forward. This is because the system will try to increase the pressure and can only do so by shifting the equilibrium forward to the side with greter number of moles, that is to the side with higher pressure. Thus the amount of C and D increases and that of A and B decreases.