Gino David PHILIPPE
| "We cannot hold a torch to light another person’s path without brightening our own." – Ben Sweetland, [1]. |
| Occupation: | Rector | ||
| Nationality: | Mauritian | ||
| Skype address: | d.g.philippe | ||
| |||
| |||
Chemistry Resources
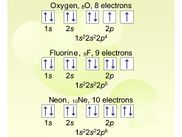
Electronic configuration
Electronic configuration : Notes for topic covered on Wednesday 17th February 2010.
Electronic configuration : Print out version notes for topic covered on Wednesday 17th February 2010.
You can read further by clicking here
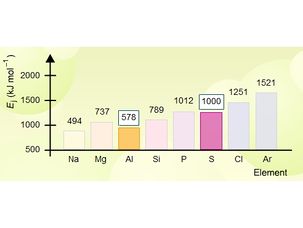
First Ionisation Energy
First ionisation energy : Notes for topic covered on Wednesday 24th February 2010.First ionisation energy : Print out version notes for topic covered on Wednesday 24th February 2010.
You can read further by clicking here
Ionic bonding
IONIC BONDING : Print out version notes for topic covered on Wednesday 2n2 March 2010.
CLICK HERE to view an animation on Fajan's Rule
Covalent bonding
Table of content for covalent bonding
1.0 The notes and animations on Covalent bonding can be seen on the Covalent page
Polar and non polar molecules
Click Here to view the notes on polar and non polar molecules
Intermolecular Forces
Click Here for the notes on intermolecular forces.
For more information on Van der Waal's and permanent dipole interactions click here
For more information on Hydrogen bondingclick here
Chemical Energetics
Click here for the notes on chemical energetics
Rate of Reaction
click here for the questions on Rate of reaction
A SIMPLE KINETICS PLAN
Hydrogen peroxide decomposes in the presence of metal oxide catalysts to form oxygen and water according to the following equation.
2H2O2 --> 2H2O + O2
You are required to plan an experiment to compare which of two metal oxides is the more effective catalyst by measuring the amount of oxygen formed during a 2 minute period immediately after a solution of hydrogen peroxide is added to a catalyst.
(a) Determine a suitable number of moles of hydrogen peroxide to be used in the experiment. (5)
(b) Suggest a suitable volume and concentration of hydrogen peroxide for the experiment. (3)
(c) Outline the procedure in full, giving details of any apparatus used. (10)
(d) Indicate any hazards or safety precautions specific to the experiment. (2)
(Total 20 marks)
Organic Chemistry
Click here For the notes on Organic Chemistry
Practical Enthalpy of solution
Click here for question
Chemical Calculation
Practice Questions and for the Answers click here
Questions on Titrations from practical papers
Chemical Equilibria
Click here for questions on Kc
Click here for questions on Kp
Click here for questions on buffer solutions
Questions NMR
Click here for questions on NMR
Notes on electrolysis
Click here for the notes on electrolysis
June 2000
Question 1
Titration of FA1 with FA2
| Final burette reading/ cm3 | 25.75 | 25.25 | 25.25 |
| Initial burette reading/ cm3 | 0.00 | 0.00 | 0.00 |
| Volume of FA 2 used /cm3 | 25.75 | 25.25 | 25.25 |
Summary iodine liberated by 25.0 cm3 of FA 1 required 25.25 cm3 of FA2
(a) Mr of Na2S2O3.5H2O = 248
Concentration of FA2 = 37.23 / 248
= 0.150 moldm-3
(b) 1000 cm3 of FA2 contains = 0.150 mol S2O32-
25.25 cm3 of FA2 will contain = 0.150 / 1000 x 25.25 moles
= 3.79 x 10-3 moles S2O32-
(c) According to equation
2 S2O32- + I2 --> S4O62- + 2I-
2 moles S2O32- react with 1 mole I2
3.79 x 10-3 moles S2O32- --> 1.895 x 10-3 moles I2
(d) 1000 cm3 FA1 contains 0.05 moles CrO42-
25 cm3 of FA1 0.05 / 1000 x 25 moles CrO42-
1.25 x 10-3 moles CrO42-
(e) 1.25 x 10-3 moles CrO42- react with 1.895 x 10-3 moles I2
1 mole CrO42- react with 1.895 x 10-3 / 1.25 x 10-3 moles I2
= 1.52 moles I2
Since 2I- --> I2 + 2e-
Number of moles of I- = 2 x 1.52
= 3.04 moles
(f) Cr3+ ions
(g) CrO42- + 8H+ + 3I- --> Cr3+ + 3/2 I2 + 4H2O