Covalent page
Covalent bonding
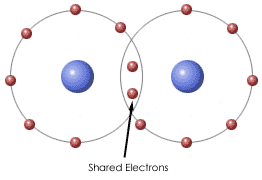
Some elements tend neither to lose nor to accept valence electrons. They achieve the stable electron configuration of a noble gas by sharing electrons.
A pair of shared electrons forms a single covalent bond. A set of atoms linked together with covalent bonds is called a molecule.
The formation of covalent bonds can be illustrated by Lewis dot structures.
Animations
1. The hydrogen molecule
2. The hydrogen Chloride
3. The Water molecule
4. The Methane molecule
5. The Chlorinemolecule
View the Lewis dot and cross diagram
Sigma and pi bond
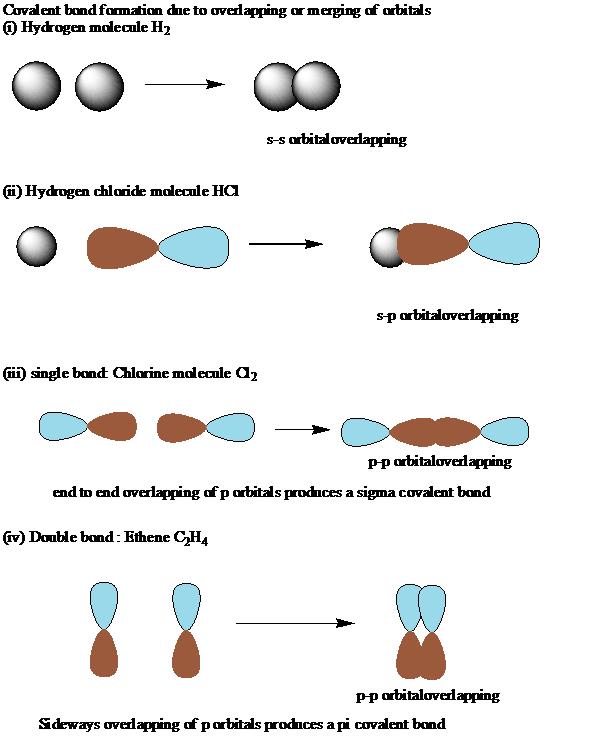
The various atomic orbitals which are pointing towards each other now merge to give molecular orbitals, each containing a bonding pair of electrons. Molecular orbitals made by end-to-end overlap of atomic orbitals are called sigma bonds.
This sideways overlap also creates a molecular orbital, but of a different kind. In this one the electrons aren't held on the line between the two nuclei, but above and below the plane of the molecule. A bond formed in this way is called a pi bond In ethene the first bond is a sigma bond while the second bond is a pi bond.
To view examples of molecules having sigma and pi bonds click here