Warming your world
Biology in Middle Schools home | |Elementary School sister project
Contents
- 1 Primary biological content area covered
- 2 Materials
- 3 Handouts
- 4 Description of activity
- 5 Lesson plan
- 6 Sample Data From Run-Through of Experiment
- 7 Discussion Questions
- 8 Potential pitfalls
- 9 Math connections
- 10 Literature connections
- 11 Connections to educational standards
- 12 Next steps
- 13 Reflections
- 14 Global Warming Facts
- 15 Citations and links
Biology In Middle Schools is a Saint Michael's College student project. Link under 'toolbox' for a printer-friendly version. Click on handouts to print full resolution versions. Please see Wikieducator's disclaimer, our safety statement, and the Creative Commons licensing in English and in legalese.
Primary biological content area covered
Students will be able to witness first-hand the fundamentals of how global warming works due to the attributes of carbon dioxide and the earth's atmosphere. This will give students a better grasp on the imminent danger caused by global warming and provide them with empirical experience to help them make better decisions about the dangers of excess and continuing carbon dioxide emissions.
Materials
- 2 thermometers
- 2 or 4 glass fish bowls
- Vaseline
- Baking soda
- Vinegar
- Heat source (lamps)
- Lids for fish bowls
Handouts
- Handouts for the experiment
Description of activity
For this activity, the students will construct two different setups to represent the differences in the ability of carbon dioxide and oxygen to absorb heat and warm the atmosphere. The mini-greenhouses will be made out of fish bowls and covered with lids. In one bowl there will be vinegar and baking soda, which will give off carbon dioxide, and in the other bowl there will also be vinegar and baking soda, but they will not be mixed so that the carbon dioxide-producing reaction won't occur. A thermometer will then be placed in each bowl and the lids will be put on (Vaseline may be used to make sure the setups are airtight). The setups will be placed under a lamp or in direct sunlight and the students will observe the changes in temperature and compare the differences.
Lesson plan
1.Turn on heating lamp so that they can begin to warm up. Make sure fishbowls are clean to prevent any residue from altering heat absorption abilities.
2. Mix 1/2 cup of vinegar with two tablespoons of baking soda in a container. The reaction will produce carbon dioxide, which you will catch in one of the fish bowls. Because CO2 is heavier than air, it will accumulate in the bowl and displace the air.
3. Cut a hole in your plastic lids just big enough for the thermometers to fit in.
4. Line the rim of the fishbowls with Vaseline. Place the container with the completed reaction from step #2 in one of the bowls. Quickly mix the vinegar and baking soda as done in step #2 and quickly cover the bowl so that all products are captured within the bowl.
5. Make sure both of the bowls are covered with the plastic covers and insert the thermometers.
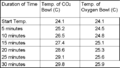
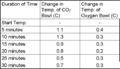
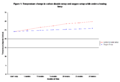
6. Observe the setups for ½ hour, recording the temperature every 5 minutes. Make sure to record the starting temperature of each setup. Pool the class data and find the average rate of temperature change per 5 minutes for each of the setups. Graph the results and answer the discussion questions.
- To demonstrate that bowl #2 does in fact contain CO2 you can drop lit matches into each bowl and the match should die instantly in bowl #2 confirming that all of the oxygen has been displaced. This can be demonstrated in extra bowls while the experiment is running.
Sample Data From Run-Through of Experiment
- Sample data from a dry run of the experiment
Discussion Questions
What is the control in this experiment? What are the independent and dependent variables? Which absorbed the most heat, oxygen or carbon dioxide? Why might too much carbon dioxide in the atmosphere be a problem? What sorts of things can we do to reduce the amount of carbon dioxide and other greenhouse gases that we let off into the atmosphere?
Potential pitfalls
Possible issues might occur if the lids are not airtight and carbon dioxide leaks out of the setup. Getting the desired amounts of carbon dioxide in the fishbowl may prove difficult. If so, light a match in the fishbowl, which will produce carbon dioxide within the closed fishbowl. Also, make sure that the light sources shine equally on the two different setups. A consistent light source from a lamp is an important variable in this experiment.
Math connections
This activity involves reading a thermometer and making quantitative observations. Other connections, such as calculating averages can also be incorporated. To do this, either pool the class data or have each group of students run several trials.
Literature connections
This activity helps students gain a basic understanding of global warming. The earth naturally goes through periods of warming and cooling, so literature on the earth's warming and cooling patterns can be introduced as well as recent statistics about the un-natural increase in atmospheric carbon dioxide and the effects that this has on the earth and its inhabitants. The general information on this topic is probably found in science textbooks.
Connections to educational standards
1.17.aa. Appropriately represent data and results in multiple ways (e.g., numbers and statistics, drawings and pictures, sentences, charts, tables, equations, simple algebraic equations, models).
1.17.bb. Use appropriate scientific, technological, and mathematical vocabulary and representations to communicate simple and complex situations.
1.17.cc. Use physical models to confirm and communicate relationships and concepts.
3.9.bb. Collect data in order to investigate and analyze how personal consumption patterns affect the sustainability of natural and human communities (e.g., buying local and imported apples in Vermont).
3.9.cc. Identify and practice ways to repair, re-use, recycle (e.g., collect and distribute leftover household paint), and design and implement a plan to monitor community resource consumption (e.g., survey community water, electric, and/or fuel use)
7.1.bb. Seek, record, and use information from reliable sources, including scientific knowledge, observation, and experimentation.
7.1.e. Explain a variety of observations and phenomena using concepts that have been learned.
7.1.f. Use either deductive or inductive reasoning to explain observations and phenomena, or to predict answers to questions.
7.5.aa. Analyze the roles and responsibilities of scientists, mathematicians, and technologists in relation to ongoing research and discoveries that impact society (e.g., the dangers and benefits of nuclear energy).
7.13.cc. Describe, model, and explain the principles of the interdependence of all systems that support life (e.g., food chains, webs, life cycles, energy levels, populations, oxygen-carbon dioxide cycles), and apply them to local, regional, and global systems.
Next steps
Students could discuss ways to reduce the amount of carbon dioxide that escapes into the atmosphere
Students could further explore and learn about the natural occurence of cabon dioxide (carbon cycle)
Discussion about the other greenhouse gases (water vapor, methane, nitrous oxide, ozone, CFCs)
Discussion about how increased global temperatures affect various ecosystems and organisms that may need specific temperatures to survive
Reflections
The experiment should run for at least half an hour so that the lamps have enough time to heat the setups. The intervals at which the temperature is recorded can be altered, but 5 minutes seems to work well.
Global Warming Facts
The most prevalent greenhouse gas is water vapor, which makes up nearly 95% of the greenhouse gases prevalent in the atmosphere (ICSC, 2008). In the past 300 years, the concentration of carbon dioxide has increased 30%, but more than half of this increase has only occurred within the past four decades (Chapin et al, 2000). We are now experiencing the sixth largest extinction event in the history of life. Humans have caused the extinction of 5% to 20% of species on Earth, which is a rate 100 to 1,000 times greater than the extinction rates that existed before humans appeared (Chapin et al, 2000). It is predicted that at the current rate of increase of greenhouse gas concentration in the atmosphere, the global temperature may rise 1.5 to 5.5 degrees Celsius within the next one hundred years (Molles, 2008). It is estimated that at least 279 species of plants and animals have already responded to global warming by moving closer to the poles and at the current rate of warming and human interference it has been predicted that over a million species will have gone extinct by the year 2050 (Gore, 2007). Increased global temperatures may also greatly impact the health of human populations. The severe weather patterns associated with elevated global temperatures, such as heat waves, flash floods, cyclones, earthquakes, drought, forest fires, and hurricanes can potentially have devastating effects on the human population. The prevalence of category IV and V hurricanes has nearly doubled within the past 3 decades (Gore, 2007). Each year, the average American contributes at least 15,000 pounds of carbon dioxide into the atmosphere. The carbon dioxide is emitted as a result of transportation, energy used in the home, and also from the processes used to produce the various products and services that we use on a daily basis (Gore, 2007).
Citations and links
[1] - EPA's site on the increased rate of carbon dioxide emissions (site also has climate change information) [2] -NASA's site on climate change [3]- The Carbon Cycle Background
Chapin, F. Stuart. 2000. Consequences of changing biodiversity. Nature, 405: 234-242.
Gore, Al. 2007. An Inconvenient Truth. Climate Crisis Online. 7 April 2009. <http://www.climatecrisis.net/thescience/>.
International Climate Science Coalition. 2008. Climate change quiz. 24 March 2009. <http://csccc.fcpp.org/question.php?csquestion_id=1>
International Panel on Climate Change. 2001. Graphics Presentations and Speeches. 24 March 2009. <http://www.ipcc.ch/graphics/gr-climate-changes-2001-wg1.htm>.
Molles Jr., Manuel C. 2008. Ecology: Concepts and Applications. Global Ecology. McGraw Hill, Boston. 548-549.