How Gas Lanterns Work
If you do much camping, have to deal with a lot of power outages or simply like to save on your electric bill, you may be quite familiar with the gas lantern.
Have you ever wondered how this device works? In this article, you'll learn all about what's going on inside a gas lantern.
Contents
Incandescence
Have you ever seen a heated horseshoe? Maybe at a blacksmith shop or on TV? If so, you know that if you get a horseshoe hot enough, it starts to glow. If you get its temperature up to 1,500 degrees F (800 degrees C) it will glow with a bright red color -- you see this temperature all the time in the coils of an electric stove, oven or toaster. In these appliances, electricity heats a coil or wire hot enough to make it glow. If you get the temperature up to about 4,500 degrees F (2,500 degrees C), you get a very bright yellow (nearly white) color. That's the temperature of a normal light bulb filament.
When something produces light because of heat, it is said to be incandescent. Anything that you heat up will glow, but different materials are better or worse at producing light as they are heated. Steel is a pretty good producer of light. Glass is a very poor producer. If you heat glass it will glow, but it gives off much less light than the same volume of steel. In the 1800s, theaters used lamps that heated a block of calcium oxide (lime) with a torch. This, by the way, is where the term limelight comes from. They used lime because it has a high melting temperature, so you can heat it to a white glow without the block melting (iron melts at 2,800 degrees F, while lime melts at around 4,600 degrees F). Lime also is a good producer of light.
How does glow-in-the-dark stuff work?
You see glow-in-the-dark stuff in all kinds of places, but it is most common in toys. My son, for example, has a glow-in-the-dark yo-yo, a glow-in-the-dark ball, a glow-in-the-dark mobile and even (if you can believe it) a pair of glow-in-the-dark pajamas! They make him easy to find at night!
If you have ever seen any of these products, you know that they all have to be "charged". You hold them up to a light, and then take them to a dark place. In the dark they will glow for 10 minutes. Some of the newer glow-in-the-dark stuff will glow for several hours. Usually it is a soft green light, and it is not very bright. You need to be in nearly complete darkness to notice it.
All glow-in-the-dark products contain phosphors. A phosphor is a substance that radiates visible light after being energized. The two places where we most commonly see phosphors are in a TV screen or computer monitor and in fluorescent lights. In a TV screen, an electron beam strikes the phosphor to energize it (see How Television Works for details). In a fluorescent light, ultraviolet light energizes the phosphor. In both cases, what we see is visible light. A color TV screen actually contains thousands of tiny phosphor picture elements that emit three different colors (red, green and blue). In the case of a fluorescent light, there is normally a mixture of phosphors that together create light that looks white to us.
Chemists have created thousands of chemical substances that behave like a phosphor. Phosphors have three characteristics:
- The type of energy they require to be energized
- The color of the visible light that they produce
- The length of time that they glow after being energized (known as the persistence of the phosphor)
To make a glow-in-the-dark toy, what you want is a phosphor that is energized by normal light and that has a very long persistence. Two phosphors that have these properties are Zinc Sulfide and Strontium Aluminate. Strontium Aluminate is newer -- it's what you see in the "super" glow-in-the-dark toys. It has a much longer persistence than Zinc Sulfide does. The phosphor is mixed into a plastic and molded to make most glow-in-the-dark stuff.
Occasionally you will see something glowing but it does not need charging. The most common place is on the hands of expensive watches. In these products, the phosphor is mixed with a radioactive element, and the radioactive emissions (see How Nuclear Radiation Works) energize the phosphor continuously. In the past, the radioactive element was radium, which has a half-life of 1600 years. Today, most glowing watches use a radioactive isotope of hydrogen called tritium (which has a half-life of 12 years) or promethium, a man-made radioactive element with a half-life of around three years.
Bring on the Heat
Probably the most common way to energize atoms is with heat, and this is the basis of incandescence. If you heat up a horseshoe with a blowtorch, it will eventually get red hot, and if you heat it enough it gets white hot. Red is the lowest-energy visible light, so in a red-hot object the atoms are just getting enough energy to begin emitting light that we can see. Once you apply enough heat to cause white light, you are energizing so many different electrons in so many different ways that all of the colors are being generated -- they all mix together to look white, as explained in one of the sections below.
Heat is the most common way we see light being generated -- a normal 75-watt incandescent bulb is generating light by using electricity to create heat. However, there are lots of other ways to generate light, some of which are listed below:
- Halogen lamps - Halogen lamps use electricity to generate heat, but benefit from a technique that lets the filament run hotter.
- Gas lanterns - A gas lantern uses a fuel like natural gas or kerosene as the source of heat.
- Fluorescent lights - Fluorescent lights use electricity to directly energize atoms rather than requiring heat.
- Lasers - Lasers use energy to "pump" a lasing medium, and all of the energized atoms are made to dump their energy at the exact same wavelength and phase.
- Glow-in-the-dark toys - In a glow-in-the-dark toy, the electrons are energized but fall back to lower-energy orbitals over a long period of time, so the toy can glow for half an hour.
- Indiglo watches - In Indiglo watches, voltage energizes phosphor atoms.
- Chemical light sticks - A chemical light stick and, for that matter, fireflies, use a chemical reaction to energize atoms.
The thing to note from this list is that anything that produces light does it by energizing atoms in some way.
Making Colors
Visible light is light that can be perceived by the human eye. When you look at the visible light of the sun, it appears to be colorless, which we call white. And although we can see this light, white is not considered to be part of the visible spectrum (Figure 2). This is because white light is not the light of a single color, or frequency. Instead, it is made up of many color frequencies. When sunlight passes through a glass of water to land on a wall, we see a rainbow on the wall. This would not happen unless white light were a mixture of all of the colors of the visible spectrum. Isaac Newton was the first person to demonstrate this. Newton passed sunlight through a glass prism to separate the colors into a rainbow spectrum. He then passed sunlight through a second glass prism and combined the two rainbows. The combination produced white light. This proved conclusively that white light is a mixture of colors, or a mixture of light of different frequencies. The combination of every color in the visible spectrum produces a light that is colorless, or white.
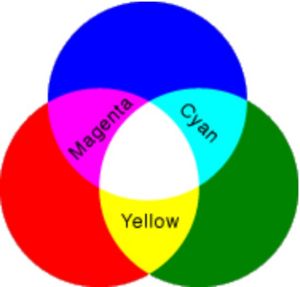
- Colors by Addition - You can do a similar experiment with three flashlights and three different colors of cellophane -- red, green and blue (commonly referred to as RGB). Cover one flashlight with one to two layers of red cellophane and fasten the cellophane with a rubber band (do not use too many layers or you will block the light from the flashlight). Cover another flashlight with blue cellophane and a third flashlight with green cellophane. Go into a darkened room, turn the flashlights on and shine them against a wall so that the beams overlap, as shown in Figure 3. Where red and blue light overlap, you will see magenta. Where red and green light overlap, you will see yellow. Where green and blue light overlap, you will see cyan. You will notice that white light can be made by various combinations, such as yellow with blue, magenta with green, cyan with red, and by mixing all of the colors together.
- Colors by Subtraction - Another way to make colors is to absorb some of the frequencies of light, and thus remove them from the white light combination. The absorbed colors are the ones you will not see -- you see only the colors that come bouncing back to your eye. This is what happens with paints and dyes. The paint or dye molecules absorb specific frequencies and bounce back, or reflect, other frequencies to your eye. The reflected frequency (or frequencies) are what you see as the color of the object. For example, the leaves of green plants contain a pigment called chlorophyll, which absorbs the blue and red colors of the spectrum and reflects the green.
Do Try This at Home
Here is an absorption experiment that you can try at home: Take a banana and the blue cellophane-covered flashlight you made earlier. Go into a dark room, and shine the blue light on the banana. What color do you think it should be? What color is it? If you shine blue light on a yellow banana, the yellow should absorb the blue frequency; and, because the room is dark, there is no yellow light reflected back to your eye. Therefore, the banana appears black.
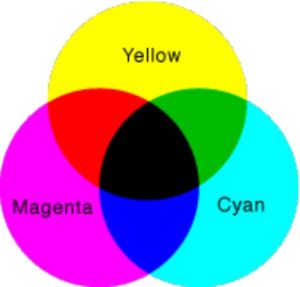
So, if you had three paints or pigments in magenta, cyan and yellow, and you drew three overlapping circles with those colors, as shown in Figure 4, you would see that where you have combined magenta with yellow, the result is red. Mixing cyan with yellow produces green, and mixing cyan with magenta creates blue. Black is the special case in which all of the colors are absorbed. You can make black by combining yellow with blue, cyan with red or magenta with green. These particular combinations ensure that no frequencies of visible light can bounce back to your eyes.
But the color scheme demonstrated in Figure 4 appears to go against what your art teacher told you about mixing colors, right? If you mix yellow and blue crayons, you get green, not black. This is because artificial pigments, such as crayons, are not perfect absorbers -- they do not absorb all colors except one. A "yellow" crayon can absorb blue and violet while reflecting red, orange and green. A "blue" crayon can absorb red, orange and yellow while reflecting blue, violet and green. So when you combine the two crayons, all of the colors are absorbed except for green. Therefore, you see the mixture as green, instead of the black demonstrated in Figure 4.
So there are two basic ways by which we can see colors. Either an object can directly emit light waves in the frequency of the observed color, or an object can absorb all other frequencies, reflecting back to your eye only the light wave, or combination of light waves, that appears as the observed color. For example, to see a yellow object, either the object is directly emitting light waves in the yellow frequency, or it is absorbing the blue part of the spectrum and reflecting the red and green parts back to your eye, which perceives the combined frequencies as yellow.
When Light Hits an Object
When a light wave hits an object, what happens to it depends on the energy of the light wave, the natural frequency at which electrons vibrate in the material and the strength with which the atoms in the material hold on to their electrons. Based on these three factors, four different things can happen when light hits an object:
- The waves can be reflected or scattered off the object.
- The waves can be absorbed by the object.
- The waves can be refracted through the object.
- The waves can pass through the object with no effect.
And more than one of these possibilities can happen at once.
Absorption
In absorption, the frequency of the incoming light wave is at or near the vibration frequency of the electrons in the material. The electrons take in the energy of the light wave and start to vibrate. What happens next depends upon how tightly the atoms hold on to their electrons. Absorption occurs when the electrons are held tightly, and they pass the vibrations along to the nuclei of the atoms. This makes the atoms speed up, collide with other atoms in the material, and then give up as heat the energy they acquired from the vibrations.
The absorption of light makes an object dark or opaque to the frequency of the incoming wave. Wood is opaque to visible light. Some materials are opaque to some frequencies of light, but transparent to others. Glass is opaque to ultraviolet light, but transparent to visible light.
Reflection
The atoms in some materials hold on to their electrons loosely. In other words, the materials contain many free electrons that can jump readily from one atom to another within the material. When the electrons in this type of material absorb energy from an incoming light wave, they do not pass that energy on to other atoms. The energized electrons merely vibrate and then send the energy back out of the object as a light wave with the same frequency as the incoming wave. The overall effect is that the light wave does not penetrate deeply into the material.
In most metals, electrons are held loosely, and are free to move around, so these metals reflect visible light and appear to be shiny. The electrons in glass have some freedom, though not as much as in metals. To a lesser degree, glass reflects light and appears to be shiny, as well.
A reflected wave always comes off the surface of a material at an angle equal to the angle at which the incoming wave hit the surface. In physics, this is called the Law of Reflectance. You have probably heard the Law of Reflectance stated as "the angle of incidence equals the angle of reflection."
You can see for yourself that reflected light has the same frequency as the incoming wave. Just look at yourself in a mirror. The colors you see in the mirror's image are the same as those you see when you look down at yourself. The colors of your shirt and hair are the same as reflected in the mirror as they are on you. If this were not true, we would have to rely entirely on other people to tell us what we look like!
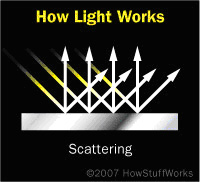
Scattering
Scattering is merely reflection off a rough surface. Incoming light waves get reflected at all sorts of angles, because the surface is uneven. The surface of paper is a good example. You can see just how rough it is if you look at it under a microscope. When light hits paper, the waves are reflected in all directions. This is what makes paper so incredibly useful -- you can read the words on a printed page regardless of the angle at which your eyes view the surface.
Another interesting rough surface is Earth's atmosphere. You probably don't think of the atmosphere as a surface, but it nonetheless is "rough" to incoming white light. The atmosphere contains molecules of many different sizes, including nitrogen, oxygen, water vapor and various pollutants. This assortment scatters the higher energy light waves, the ones we see as blue light. This is why the sky looks blue.
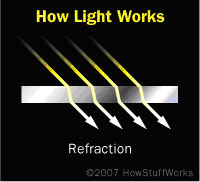
Refraction
Refraction occurs when the energy of an incoming light wave matches the natural vibration frequency of the electrons in a material. The light wave penetrates deeply into the material, and causes small vibrations in the electrons. The electrons pass these vibrations on to the atoms in the material, and they send out light waves of the same frequency as the incoming wave. But this all takes time. The part of the wave inside the material slows down, while the part of the wave outside the object maintains its original frequency. This has the effect of bending the portion of the wave inside the object toward what is called the normal line, an imaginary straight line that runs perpendicular to the surface of the object. The deviation from the normal line of the light inside the object will be less than the deviation of the light before it entered the object.
The amount of bending, or angle of refraction, of the light wave depends on how much the material slows down the light. Diamonds would not be so glittery if they did not slow down incoming light much more than, say, water does. Diamonds have a higher index of refraction than water, which is to say that they slow down light to a greater degree.
One interesting note about refraction is that light of different frequencies, or energies, will bend at slightly different angles. Let's compare violet light and red light when they enter a glass prism. Because violet light has more energy, it takes longer to interact with the glass. As such, it is slowed down to a greater extent than a wave of red light, and will be bent to a greater degree. This accounts for the order of the colors that we see in a rainbow. It is also what gives a diamond the rainbow fringes that make it so pleasing to the eye.
Rainbows in Soap Bubbles
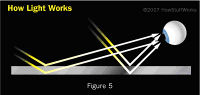
Have you ever wondered why soap bubbles are rainbow colored, or why an oil spill on a wet road has rainbow colors in it? This is what happens when light waves pass through an object with two reflective surfaces. When two incoming light waves of the same frequency strike a thin film of soap, as seen in Figure 5 below, parts of the light waves are reflected from the top surface, while other parts of the light pass through the film and are reflected from the bottom surface. Because the parts of the waves that penetrate the film interact with the film longer, they get knocked out of sync with the parts of the waves reflected by the top surface. Physicists refer to this state as being out of phase. When the two sets of waves strike the photoreceptors in your eyes, they interfere with each other; interference occurs when waves add together or subtract from each other and so form a new wave of a different frequency, or color.
Basically, when white light, which is a mixture of different colors, shines on a film with two reflective surfaces, the various reflected waves interfere with each other to form rainbow fringes. The fringes change colors when you change the angle at which you look at the film, because you are changing the path by which the light must travel to reach your eye. If you decrease the angle at which you look at the film, you increase the amount of film the light must travel through for you to see it. This causes greater interference.
Everything we see is a product of, and is affected by, the nature of light. Light is a form of energy that travels in waves. Our eyes are attuned only to those wave frequencies that we call visible light. Intricacies in the wave nature of light explain the origin of color, how light travels, and what happens to light when it encounters different kinds of materials.