Fruit fly X-ing
Biology in Middle Schools home | |Elementary School sister project
Contents
Biology In Middle Schools is a Saint Michael's College student project. Link under 'toolbox' for a printer-friendly version. Click on handouts to print full resolution versions. Please see Wikieducator's disclaimer, our safety statement, and the Creative Commons licensing in English and in legalese.
Primary biological content area covered
- using compound microscopes
- using dissecting microscopes
- learning the basic concepts of genetics
- practice taking and keeping organized notes
Materials
Teacher Materials
- Anesthetizing Agent (Fly-Nap or alternative freezing method or CO2)
- Red eyed female (wild type) Drosophila melanogaster
- Red eyed male (wild type) Drosophila melanogaster
- White eyed female (mutants) Drosophila melanogaster
- White eyed male (mutants) Drosophila melanogaster
- Fruit Fly feeding medium
- Vials to contain the specimens
Group Materials
- Compound microscope
- Dissecting microscope
- Fruit fly slides (of teacher's choice)
- Vial of each fly type for viewing under dissecting microscope
Individual Student Materials
- Notebook
Handouts
Written instructions that students could use during this activity are linked here.
Description of activity
Students will learn the understanding of what a gene and a chromosome are. They will understand the difference between homologous and heterozygous traits as well as the difference between genotype and phenotype. Students will be able to predict the possible traits and their probabilities following the sexual cross of flies with different characteristics.
Lesson plan
- Title of Lesson: Fruit Fly X-ing
- Length of time needed to complete: Time length is flexible depending on teacher’s interest. For students to observe full life cycles and crosses, one class per week for three weeks is recommended.
- Age/Grade: 7th/8th graders
- Teacher Content Knowledge: Teacher needs to be aware of the fruit fly life cycle, and understand how to culture flies in the lab. Also teacher should be familiar with general genetic concepts including: homozygous/heterozygous traits, genotype/phenotype, genes, alleles, chromosomes, Punnett squares for calculating probability, inheritance, and also general dissecting and compound microscope techniques.
- Resources/Materials:
- For teacher: anesthetizing agent (Fly-Nap or alternative freezing method), red eyed female (wild type) Drosophila melanogaster, red eyed male (wild type) Drosophila melanogaster, white eyed female (mutants) Drosophila melanogaster , white eyed male (mutants) Drosophila melanogaster, fruit fly feeding media, vials to contain the specimens. (If the CO2 method is going to be used, you will need a Mason jar, with two lids, Alka-seltzer, and cheese cloth)
- Per group: lab handout, dissecting microscope, compound microscope, fruit fly slides (of teacher's choice), vial of each fly type for viewing under dissecting microscope, notebook for recording observations/data.
- For teacher: anesthetizing agent (Fly-Nap or alternative freezing method), red eyed female (wild type) Drosophila melanogaster, red eyed male (wild type) Drosophila melanogaster, white eyed female (mutants) Drosophila melanogaster , white eyed male (mutants) Drosophila melanogaster, fruit fly feeding media, vials to contain the specimens. (If the CO2 method is going to be used, you will need a Mason jar, with two lids, Alka-seltzer, and cheese cloth)
- Rationale and Context: This assignment will be helpful to students to understand how inheritance works through the manipulation of various phenotypes. It will help them to understand basic concepts of genetics and see them unfold in living organisms.
- Essential Question: How does inheritance work?
- Focusing Question: What are the roles of dominant and recessive alleles in determining phenotype?
- Vital Results: Students understand how phenotype is determined by the parent generation’s genotype, the difference between heterozygous and homozygous traits, as well as the ability to predict phenotypes of future generations based on probabilities from creating Punnett squares.
- Vocabulary:
- 1. Homozygous: possessing two identical alleles (genes).
- 2. Heterozygous: possessing two different alleles (genes).
- 3. Gene: a discrete unit of hereditary information (DNA) located on a chromosome that code for a trait.
- 4. Allele: an alternate form of a gene.
- 5. Chromosome: an organized structure of DNA and protein found in a cell.
- 6. Genotype: the complete set of hereditary information, an organism’s genetic makeup.
- 7. Phenotype: a characteristic or trait expressed by an organism.
- 8. Punnett Square: a grid (square) used to predict all possible outcomes of gametes in a genetic cross.
- 1. Homozygous: possessing two identical alleles (genes).
- Procedure:
- Obtaining Flies: Flies, vials, feeding medium, and meshwork can be purchased online at Carolina Biological Supply (http://www.carolina.com/home.do).
- Experiment:
- 1. Obtain vials of red eyed males and white eyed females.
- 2. Administer Fly Nap, or other anesthetic agent to the tubes as directed by the bottle.
- To use the CO2 method, you will need to take the inner cap portion out of both lids, glue the two lids together so that both lids could still be screwed on to their jars. Place water in the Mason jar, and place a piece of cheese cloth over the opening. Screw the lid on. When you are ready to knock out the flies you can place two Alka-seltzer tablets into the water, and screw the top back on with the cheese cloth in place. Now place the flies on top of the cheese cloth. The CO2 gasses that are being released from the jar will knock out the flies so they can be observed.
- Obtaining Flies: Flies, vials, feeding medium, and meshwork can be purchased online at Carolina Biological Supply (http://www.carolina.com/home.do).
- 3. After the flies are anesthetized they can be removed from the vials for observation. At this time allow all the students to observe the flies and their eye color under the dissecting scopes. Now would also be a good time for the students to learn how to determine the sex of the flies.
- Determining sex: The female’s abdomen tip is more elongated than the male’s. Also the abdomen is more rounded in males compared to females. And finally, sex combs are found on front legs of male flies.
- 4. After observations, the students should create crossing vials by placing 10 red eyed males, and 10 white eyed females (P generation) together with feeding medium and meshwork.
- 5. Wait approximately 10 days, and teacher should remove all flies (and euthanize), but be careful to leave behind larva, so as not to mix up student’s counting of progeny.
- 6. After larva reaches adult form have students observe results from first cross (F1 generation) recording sex and eye color, using the method described in step 2, into a table.
- 3. After the flies are anesthetized they can be removed from the vials for observation. At this time allow all the students to observe the flies and their eye color under the dissecting scopes. Now would also be a good time for the students to learn how to determine the sex of the flies.
- NOTE: If more time is available more crosses could be observed in this experiment (F2, F3, etc.). Additionally, different mutations could be looked at, such as functionality of wings. Also, while waiting during larval stage, it would be helpful to view slides of fruit flies using compound microscopes.
- Punnett Squares:
| Punnett Square | |
| Wikipedia has an article on this subject.
Visit Punnett Square for more in depth information |
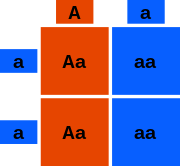
In Punnett squares, the heterozygous/heterozygous and dominant/recessive characteristics are important. In the example above we are crossing a homozygous recessive individual (green) with a heterozygous dominant (red) individual. The heterozygous is denoted as Aa and the homozygous is denoted aa. Each letter represents an allele. The letters on the outside of the box are the P generation and the letters on the inside are the possible genotypes of the offspring. To determine which letters go inside the squares you take the letters bordering it and put them inside. For example, in the top left square we put a letter A and a letter a, because on the top border of the box there is an A and on the left side border there is an a. Because the two boxes on the left hand side contain the dominant allele A, then they exhibit the red dominant color. The two boxes on the right side have only the recessive allele a, so they are green in color. So, to determine probability there is a 2/4 chance (or 50%) that the offspring will be green, and a 2/4 chance the offspring will be red.
- Lab Report for Students:
The lab report should be types and include four main sections.
- 1. Pre-lab Questions-
- A. Why are fruit flies a model organism for genetic study?
- B. Explain the differences between male and female specimens.
- C. Define the following terms: homozygous, heterozygous, gene, allele, chromosome, genotype, phenotype.
- A. Why are fruit flies a model organism for genetic study?
- 2. Hypothesis- Take a stance on which eye color (red or white) you believe to be dominant and which you think is recessive. Also make Punnett squares to show the possible outcomes that would result in each case.
- 3. Data- Organize your data into a neat and well labeled table (don’t forget legends and an informative title).
- 4. Conclusions- Was your hypothesis correct? Why or why not? Does your data support your Punnett square predictions (i.e. does the ratio of your Punnett square correspond to the ratio in your data)?
- 1. Pre-lab Questions-
- Grading: Grading is based on the expectations and requirements set by the teacher and school.
Suggestions include: Organization, on time, neatness, If-then hypothesis, accuracy of Punnett square (in terms of procedure).
- Clean up: students should wash hands and disinfect benches after each lab session. On last day of activity materials can be thrown away in trash along with euthanized flies. Flies can be euthanized by prolonged exposure to freezing temperatures (overnight) or prolonged exposure to fly nap.
Potential pitfalls
- If the flies are not fully knocked out they can fly away
- this can be avoided by following the directions on the Fly Nap bottle
- Incomplete separation from parent (P) generation from F1 generation can result in unwanted crossing and your data will be compromised
- this can be controlled by removing all of the P generation 10 days after the initial cross
Math connections
- Punnett Squares (calculating probability of offspring outcome)
see lesson plan for how to construct and properly fill in a Punnett square
Connections to educational standards
Vermont Framework of Standards and Learning Opportunities:
Grade 5-8: Science, Mathematics, and Technology Standards
Scientific Method
- 7.1 Students use scientific methods to describe, investigate, and explain phenomena and raise questions in order to:
- Generate alternative explanations - hypotheses - based on observations and prior knowledge
- Design inquiry that allows these explanations to be tested;
- Deduce the expected results;
- Gather and analyze data to compare the actual results to the expected outcomes; and
- Make and communicate conclusions, generating new questions raised by observations and readings.
- 7.1.bb. Seek, record, and use information from reliable sources, including scientific knowledge, observation, and experimentation;
- 7.1.cc. Create hypotheses to problems, design their own experiments to test their hypothesis, collect data through observation and instrumentation, and analyze data to draw conclusions; use conclusions to clarify understanding and generate new questions to be explored;
- 7.1.dd. Describe, explain, and model, using evidence that includes scientific principles and observations;
- 7.1.gg. Propose, recognize, and analyze alternative explanations; and
- 7.1.ii. Work individually and in teams to collect, share, and present information and ideas.
Statistics and Probability Concepts
- 7.9 Students use statistics and probability concepts. This is evident when students:
- 7.9.dd. Find all possible combination, arrangements, and/or permutations within given constraints; make predictions based on experimental or theoretical probability; recognize equally likely outcomes and determine the probabilities of events; predict the results of a series of trials once the probability for one trial is known
The Human Body
- 7.14 Students demonstrate understanding of the human body heredity, body systems, and individual development and understand the impact of the environment on the human body. This is evident when students:
- 7.14.aa. Describe how genetic information is passed through reproduction (e.g., genes, traits, chromosomes)
Website: http://education.vermont.gov/new/pdfdoc/pubs/framework.doc
Next steps
Depending on time available, the teacher can choose to do more activities
- Look at multiple generations after the F1 generation
- Look at multiple phenotypes (ex. wing mutations)
- Practice/construct dihybrid crosses rather than monohybrid crosses
- The students can draw what they see under the compound microscope, using the slides the class has (they can use different magnifications and different slides)
Citations and links
- Ashburner, M., Wright, T.R.F (editors). 1978. The Genetics and Biology of Drosophila, 2b. Academic Press, NY, pp. 127-135.
- Cakir, S., Bozcuk, A.N., 1999. Longevity in some wild type and hybrid strains of Drosophila melanogaster. Turkish J. Bio. 24:321-329.
- Demerec, M., Kaufmann, B.P. 1996. Drosophila guide: introduction to the genetics and cytology of Drosophila melanogaster, Tenth ed. Carnegie Institution of Washington, Washington, D.C., pp. 1-45.
- Herr, N. 2008. The Sourcebook for Teaching Science: Strategies, Activities, and Additional Resources. Jossey-Bass Teacher, CA, pp. 517-519, 525.
- Michaels, S., Shouse, A.W., Schweingruber, H.A. 2008. Ready, Set, Science!: Putting Research to work in K-8 Science Classrooms. The National Academies Press, Washington, D.C., pp. 153-156.
- Pierce, B.A. 2006. Genetics: A Conceptual Approach, 2nd ed. W.H. Freeman and Co., NY, pp. 87, 610.
- Unknown, Nasonia genetics-A jewel of a Wasp [Internet]. 2009. [cited 2009 Feb. 20]. Available from http://www.biologycorner.com/worksheets/nasonia.html.
An Introduction to Drosophila Melanogaster', http://biology.arizona.edu/sciconn/lessons2/Geiger/intro.htm