Antarctica/Exploration ICEBLOCK/Teachers Backpack/Experiments with SALTY and FRESH WATER
- You could try starting with this simple EXPERIMENT: FLOATING EGGS suitable for NE (new entrants) to Year 8 to begin the process of understanding properties of salt water.
EXPERIMENT: LIFTING ICE WITH A MATCHSTICK
- Salt is spread on roads in the winter to melt the ice. The salty water that is left does not freeze until the temperature is well below 0degreesC. This simple experiment explores this property in an effective way.
EQUIPMENT
- A bowl of water, an icecube, a matchstick and some salt.
PROCEDURE
- Float the icecube in the bowl of water and lay the matchstick carefully on the top of the cube. Encourage the children to predict what they think will happen when some salt is sprinkled on top of the icecube and explain why they think this.
- Sprinkle a little salt around the matchstick. Soon it will be frozen into the icecube and you will be able to use the matchstick to lift the ice.
As the children observe what happens, encourage them to explain the reasons for this.
NOTES FOR TEACHERS: Here's how it works. When you sprinkle salt onto the icecube it makes the ice around the matchstick melt, because salt water freezes at a lower temperature than fresh water. But no salt falls under the matchstick so it becomes frozen onto the ice. That's why you can lift the ice with the matchstick.
ADDED NOTE: Adding antifreeze to the water in car radiators is another way to stop water freezing. A mixture of the two liquids will not freeze until minus 30degreesC.
EXPERIMENT: COMPARING SALT WATER TO FRESH WATER
- Sea water is always salty but in some places where it rains a lot (like near the Equator) or where land ice melt meets sea (like in Antarctica), the sea is less salty than elsewhere. This experiment will let children discover what happens when a little salt water meets lots of fresh water and when a little fresh water meets lots of salt water.
Encourage the children to predict what they think will happen and explain possible reasons for this predicted water behaviour (what experiences or previous observations are they drawing on to make their predictions?).
EQUIPMENT
- salt
- water
- green and blue food colouring
- two droppers
- two clear small containers (about 30ml or two similar small glasses)
- two clear medium containers (about 250ml or two similar glass jars)
PROCEDURE
- Pour water into the two medium containers until they are half full.
- Pour a tablespoon of salt into one of the containers and stir until the salt dissolves. Label this container "Salt Water" and the other one "Fresh Water".
- Pour some of the salty water into one of the small containers until it is 3/4 full.
- Add green colouring to the small cup of salty water until it is DARK GREEN. Label this container "Salt Water".
- Pour fresh water into the other small cup until it is 3/4 full, colour it LIGHT BLUE and label it "Fresh Water".
- Now use the dropper to add drops of GREEN salt water to the CLEAR fresh water.
- Observe and draw what happens. Add drops of BLUE fresh water to the CLEAR salt water. Observe and draw what happens. Use colour pencils to draw the location of the salt water and the fresh water in your diagrams.
EXPLAIN
- What happened to the salt water when it was added to the fresh water? Why do you think this occurred?
- What happened to the fresh water when it was added to the salt water? Why do you think this occurred?
- What do you think happens when fresh water runs into ocean water? Why?
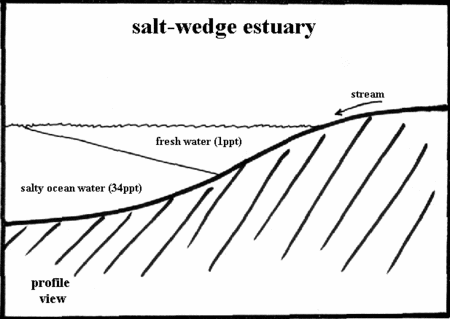
NOTES FOR TEACHERS: The fresh water just floats on top of the salt water because salt water is denser. The place where fresh water meets the salty sea water is called an Estuary.
- Click here for Extended Experimental Exploration of Fresh and Salt water More Fun with Salt and Fresh Water