Human earth shapers/ETHS101/Rock Record/Relative and absolute ages
Contents
Relative and Absolute Ages
Fossils are not only interesting, intriguing and beautiful, they can also be very useful
Using the fossil succession spatially
Canals were dug all over Europe and England in the 1700s to transport large volumes of raw materials and goods required for the new Industrial Revolution. In England, William Smith was building canals.
He realised that some strata were easier to dig than others. He noted that the strata contained fossils and that the fossils succeeded each other in a systematic way.
Using these ideas he could predict the location of strata and plan his canal routes to be the most cost effective. He made the first geological map, published in 1815.
Using fossil succession across time (temporally)
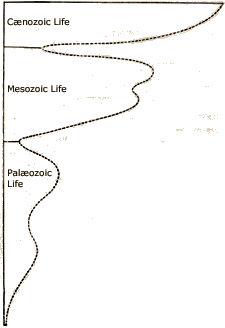
In the early to middle 1800s people began to divide rocks and fossils into different groups and understand their relationship to one another in relative time. Fossils were divided into the Paleozoic era (Palaeozoic = old life), Mesozoic era (=middle life) and Caenozoic era (Cenozoic = young life).John Phillips (the nephew of William Smith) drew the elegant to show the abundance of fossils through time.
The curve illustrates the point that Greg made in his lecture on fossils: that as you go back in time there are fewer fossils. In this curve, you also see that there are two necks or times when there are very few fossils between the main eras. These breaks coincide with mass extinctions.
In a relative sense, fossils can provide information on biozones in the rocks or biostratigraphy.
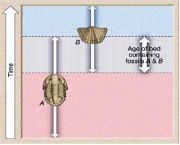
To be useful for relative dating, fossils must be common and widespread. Pollen, that Greg mentioned in his video are excellent biozone fossils. Individual fossil species that only lived for a short duration can be useful as index fossils. More often it is a group of fossils, called a fossil assemblage, used together that provide a better constraint on the relative ages of rocks. Here is a diagram on how this works:
- If rocks only contain the trilobite fossil (A) they belong to the oldest rocks in these strata.
- If rocks only contain the brachiopod shell fossils (B), they are the youngest rocks in the strata.
- If rocks contain both trilobites and brachiopods we can constrain the age of the rocks to older than trilobite-only strata and younger than brachiopod-only strata (A and B).
(The picture may be clearer than the words!) This will be a useful concept for your first assessment task (1b)
Absolute Ages
Radioactivity was discovered in the 1890s by Henri Becquerel and was studied by Wilhelm Rotingen and Pierre and Marie Curie. Not long after, in 1907, Bertram Boltwood used the steady rate of radioactive decay to find out the age of rocks.
Radioactive or radiometric dating is now the standard technique used to tell us how old rocks are. The oldest rocks on Earth are the Acasta Gneiss in Canada formed 4,030 million years ago. The oldest minerals are zircons from the Jack Hills in Western Australia. Radiometric dating indicates that the zircon crystallised 4,375 million years ago. Earth is 4,527 million years old. This age is estimated from applying radioactive dating to meteorites, which probably formed at the same time.
Atoms, elements and radioactivity
To understand how Radioactive or Radiometric dating working, we need to visit some basic principle of Chemistry. Below are a set of terms that are useful to know before we begin to explain the process of Radiometric Dating.
- Atoms
- An atom is the smallest constituent unit of ordinary matter that has the properties of a chemical element.
- Protons
- A proton is a subatomic (part of/make up) particle of an atom. Protons have a positive charge.
- Neutrons
- A Neuton is also a subatomic particle of an atom. Neutrons have no charge.
- Nucleus
- Is the centre of an atom consisting of protons and neutrons.
- Isotope
- Isotopes are variations of an element. A variation occurs when two of the same elements have different atomic values. This means the same number of protons but a different number of neutrons.
- Atomic Number
- All atoms of a given element have the same number of protons
- Mass Number
- The number of protons + neutrons
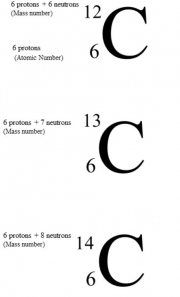
If we look at the element carbon, which has the chemical symbol C. We can observe in this case we have an atomic number of 6. This means 6 protons. There is also the mass number of 12. This is the 6 protons + the 6 Neutrons.
When Geologist are using radiometric dating techniques, they are looking to radioactive signatures. radioactivity is the spontaneous decay of an atom of one element into a different element or a different isotope of the same element.
For each radioactive isotope, decay occurs at a constant rate. This is why radioactive isotopes make excellent clocks.
The time it takes for exactly half the isotopes in a group to decay is called the half-life. Below is a video explaining Radioactivity dating.
When an organism stops breathing and eating it stops taking up 14C. 14C will start to decay. It takes around 5,700 years for half of the 14C to turn into 12C (+13C). This is good for archeology (limited to stretching back around 40,000 years), but does not have a long enough half-life for deep time.
For rocks we need isotopes that decay at a much slower rate. Uranium (U) isotopes decay to lead isotopes (Pb) over much longer half-lives and so are good for dating rocks:
238U (decays to) 206Pb, Half-life= 4,510 million years 235U (decays to) 207Pb, Half-life= 713 million years
These are the isotopes measured for U-Pb radiometric dating.
When minerals crystallise, all atoms and ions are locked into the crystal structure: a closed system (in an ideal world).
As radioactive isotopes decay, the products of the decay are preserved in the mineral crystals.
It is possible to measure the numbers of parent and daughter atoms using a mass spectrometer
Knowing the experimentally determined half-life for particular decay processes, it is possible to calculate how long radioactive decay has been occurring in the mineral = how long ago the mineral crystallised!
Geological Time
Geological time is long. Here is a short video to give you a sense for geological time using a "timeline" on the beach. The timeline is 32 m (= 4,570 million years) long and I move along it to different major events in Earth's geological history. Not much happens for a very long time! But you may be surprised to find that life is present very early on.
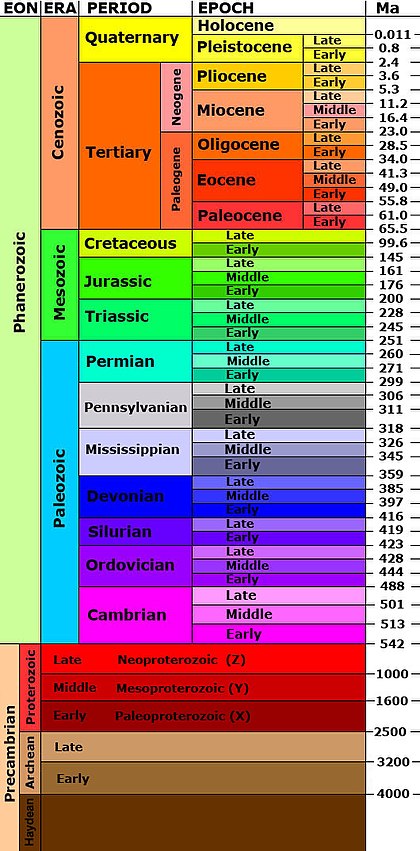
Geological time is divided into eons, eras, periods and epochs. Many of these were established first from the relative ages of fossils. Radiometric dating has added the absolute ages (on the right) on the geological time chart below (Ma = million years ago).
Note: As the chat shown here is American, the period between 359 and 299 million year ago is divided into Mississippian and Pennsylvanian. This same time period is known as Carboniferous in English and Australian charts. Also this time chart is not to scale!
The older rocks have fewer subdivisions. This is because they contain few if any fossils.
For a more details Geological time scale, the International Commission on Stratigraphy provides a great resource.