Visit the page on Hess's Law
Introduction
Some energy changes can be calculated directly from experiment while others cannot.
Using the Bomb Calorimeter, it is possible to calculate the enthalpy change of combustion of fuel such as ethanol, but it is not possible to calculate the standard enthalpy change of formation of ethanol.
Some energy changes can only be calculated indirectly using Hess's Law.
Hess's Law
Hess' Law states that the heat evolved or absorbed in a chemical process does not depend on the route followed by a reaction but depends only on the initial state of the reactants and the final state of the products. The enthalpy change is the same whether the process takes place in one or in several steps. This is also known as the law of constant heat summation.
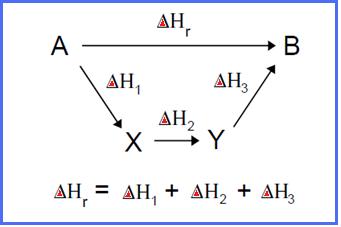 If route 1 is the change from state A to state B directly, indicated by ^Hr then route 2 is from state A to state B but passing through state X and state Y
If route 1 is the change from state A to state B directly, indicated by ^Hr then route 2 is from state A to state B but passing through state X and state Y