User:Phaello/sandbox/Chemistry/Introduction to Periodic Table
Introduction to Periodic Table
The number of protons in an atom is always equal to the number of electrons in the same atom, and some of the things we noted were:
- Protons have a positive charge
- electrons have a negative charge
- That means that if an atom has 2 protons (+2), there will be 2 electrons (-2)
- so the atom is neutral – because the number of positive charges is the same with that of negative charges.
If you know the number of protons in an atom, automatically you will know the number of electrons. If you are told that an atom has 2 protons, you should be able to tell that the number of electrons is 2 – because the atom has to be neutral.
Electrons in atoms are placed on shells. The electron shells around the nucleus can only take a certain number of electrons. The first shell in any atom can take a maximum of 2 electrons; the second shell can take a maximum of 8 electrons, the third shell can take a maximum of 18.
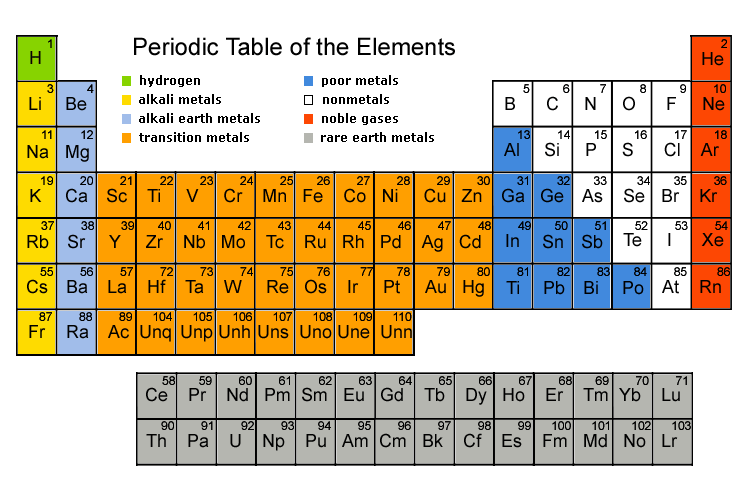
The number of protons in an atom is called Atomic Number.
Atoms in the periodic table are arranged according to the atomic numbers (i.e. number of protons). You have been provided with a copy of the periodic table, use it to do the following activity. Note that the number given against each atom symbol tells you the atomic number (i.e. number of protons).
| Starting from Hydrogen up to Argon make neat drawings of the atoms showing protons in the nucleus and the shells around it, and fill electrons in the shells. I repeat: these must be neat drawings, nice circles should represent shells. Work together, but each one must make his/her own drawings.
|
After making the drawings you should be able to see how many electrons each of the atoms has on the outermost shell. We will discuss these in the next class.
Ha e le mona u qetile mosebetsi o ka holimo, Please click here to read more about the Periodic Table.