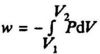The1stLawofThermodynamicsLesson4
THE JOULE - THOMSON EXPERIMENT
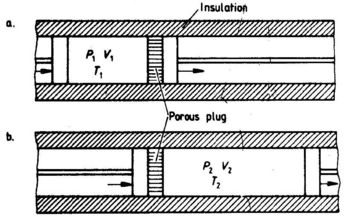
The Joule experiment (see Fig. 1.5) shows that ΔT = 0 when an ideal gas expands under the condition w = O. By careful measurements Joule and Thomson found a small temperature change, usually negative, when a real gas expanded adiabatically through a porous plug. The experimental arrangement is shown in Fig. 4.1.
Fig. 4.1. The Joule - Thomson experiment .Adiabatic expansion of a real gas through a porous plug. (a) Initial state. (b) Final state. The pressures PI and P2 are kept constant during the expansion. The work supplied to the gas on the left hand side of the porous plug is P1VI,and the work carried out by the gas on the right hand side of the porous plug is P2V2. The net work received by the gas is:
w = P1V1-P2V2 (4.2)
The process is adiabatic, q = O. From the first law we have:
ΔU =U2-U1= w = P1V1-P2V2
which gives:
U2+P2V2=U1+P1V1
From the definition of enthalpy (eq. (4.11)) we obtain:
H2 = H1 (4.3)
This means that the enthalpy is constant during the expansion. Joule and Thomson observed that the pressure change, ΔP = P2 - PI, gave a change in temperature, ΔT = T2 - T1. For most gases at room temperature one observes a positive ratio ΔT/ΔP. The differential:
is called the Joule - Thomson coefficient. The total differential, dH, can be expressed by the Joule ¬Thomson coeffisient. For the function H = f(P, 1), the total differential is expressed by eq. (2.12):
The last term is equal to CpdT (compare eq. (3.2)). For the Joule - Thomson experiment dH = 0, see eq. (4.3). Dividing eq. (2.12) with dT for dH = 0 we obtain:
or:
Thus the change in enthalpy with changes in P and T is equal to:
dH = - CpμdP + CpdT (4.6)
This equation is used in calculations of the enthalpy of real gases at high pressures when experimental values are known for the Joule - Thomson coefficient. Joule - Thomson expansion is important in refrigeration and in the liquefaction machine for condensing gases to liquids at very low temperatures.
FUNDAMENTAL EQUATIONS
The first law of thermodynamics:
dU=dq+dw(4.7) (4.7)
For a cyclic process:
The reversible pressure - volume work:
Isothermal reversible pressure - volume work supplied to an ideal gas:
w= -nRT ln (V2/V1) (4.10)
Definition of enthalpy:
H= u+pv (4.11)
Heat capacities:
Cv= (∂U/∂T)y (4.12)
Cp = (∂H/∂T)p (4.13)