Redox
Contents
Basic Reduction-Oxidation
Oxidation
Oxidation is the loss of electrons
Reactant that is oxidaised is called the REDUCTANT
So the reductant reduces other reactant, while itself oxidised
Increase in oxidation number
e.g. Fe to Fe2+ goes from having oxidation number of 0 to 2.
Reduction
Reduction is the gain of electrons
Reactant that is reduced is called the OXIDANT.
The oxidant oxidizes other reactant, while itself is reduced
Decrease in oxidation number
e.g. In Fe2+ to Fe goes from having oxidation number of 2 to 0.
Oxidation Number
Oxidation number is written with the sign before the whole number. e.g. +1 or -3
N.B. this is different to ions which have the number before the sign e.g. Al3+ or Zn2+
Oxidation number does not necessarily have to be a whole number
Oxidation–Reduction and Electrochemistry
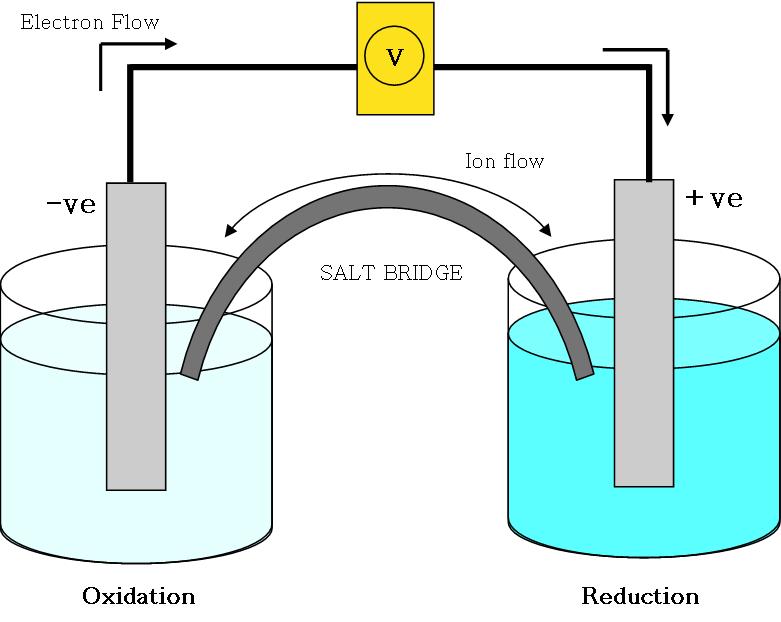
Losses electrode: oxidation = negative electrode = ANODE
Gains electrode: reduction = positive electrode = CATHODE
Salt bridge must be present to complete the circuit; it links the half-cells Salt bridge allows the flow of ions so that the change in each half-cell remains balanced.
Electrochemical Cell
Electrons flow around the circuit, which is completed by the salt bridge.
Ion is transferred through the salt bridge from one to another.
Electrons are produced and transferred through an external circuit to the other part of the cell where a reduction reaction take place, accepting the electrons.
Summary
When two half-cells are connect to form an electrochemical cell.
* The half-cell with the more positive standard electrode potential becomes the positive terminal of the cell.
* The half-cell with the more negative standard electrode potential becomes the negative terminal of the cell.
* Electrons flow in the external circuit from the negative to the positive terminal. (Oxidation to Reduction)
Ecell is the difference between the standard electrode potentials of the two half-cells.
Standard Electrode Potentials
To make things easier, a reference half cell is used to measure all the others against it, so that a common reference point is made.
A standard hydrogen half-cell is chosen as the reference. The half-reaction in this half-cell is 2H+ (aq) + 2e- --> H2 (g)
Standard Condition
* Electrical potentials vary with temperature, so far all cells a temperature is defined to 298K (25°C) (K is another unit of temperature)
* All solutions have a concentration of 1.00 mol L-1.
* All gas pressure at 1 bar or 100kPa (kilopascals)
Potential of the standard hydrogen half cell is defined as 0.00V, a value chosen for convenience.
Cell Diagrams
Instead of drawing the diagrams all the time to show the reaction between the two half cells, a cell diagram can be used instead.
The cell diagram consists mainly of two parts. The parts are oxidation and reduction.
The left hand side is oxidation and the right hand side is the reduction
A basic form is like this: electrode (s) /solution (aq) // solution (aq) / electrode (s)
NB: the state of each element/ion should be included in the cell diagram
A slash, / , is used to separate between different ions or elements with different states
Double slash, // , is used to separate between the two half cells. (separating between reduction and oxidation)
A comma, , ,can used to separate between the same states.
I.e. electrode (s) / solution (aq) , solution (aq) / gas (g) // solution (aq) / electrode (s)
The order of the aq or g does not matter