Microbial Metabolism
This is based on New Zealand Qualification Authority Unit 8023 entitled "Demonstrate knowledge of microbial metabolism."
1.1
The description outlines the nutritional requirements of microorganisms.
To find out the nutritional requirements of bacteria follow this link [1] and read slides 2,3,4.
A cells need for carbon, as a nutrition source is universal, as is an energy source to facilitate metabolism which feeds the bioenergetic pathways (the glycolosis and the krebs cycles). This is true for animal cells, plant cells and bacterial cells alike.
Micro organisms have a wide variety of nutritional and environmental requirements, depending on the charecteristics of individual species.
While carbon is required in the largest amounts by micro-organisms, water is also essential in the living environment. As this essential water is always present in the micro-organisms living environment, it can be used as a nutrition source, from which hydrogen and oxygen can be obtained. The opposite may also be true, many species living in hostile environments without readily available water derive it via Glycolysis which yields water in the Enolase phase when 2-phosphoglycerate is turned to phosphoenolypyruvate.
Also required in smaller amounts depending on type of micro organisms, are elements such as nitrogen and sulfur, and various inorganic salts. Some of micro-organisms uses inorganic as sole nutritional requirements and some uses higher complex organic compounds. They uses carbon, oxygen, nitrogen, water in one way or other way for their nutrition .e.g. hydrogen and oxygen forming organic and inorganic compound which is good source of nutrient. Nitrogen is an essential element for synthesis of enzymes and other cellular proteins as well as nucleic acids ,RNA and DNA. Nitrogen is usually obtained from nitrogenous compounds such as nitrates and ammonium compounds in the environment. Phosphorous is also essential for synthesis of nucleic acids and adenosine triphosphate (ATP), a compound that is extremely important for energy storage and transfer. Oxygen, while not essential to respiration aids ATP production. When unable to use oxadative phophorylation pathways many organisms instead resort to anaerobic respiration turning pyruvate into lactate or even ethanol and carbon dioxide!
Inorganic ions such as sulphate and phosphate can also supply the main elements needed by microorganisms.Other mineral elements are also needed but in small amounts(as a few milligrams per litre)for example: Zinc, Copper, Manganese, Molybdenum and Cobalt.These mineral elements are required to activate enzymes for example molybdenum is required by nitrogenase,the enzyme that converts the atmospheric nitrogen gas to ammonia during "Nitrogen Fixation".
Image taken by Radheyshyam N Bhagat UCOL National Diploma in Science 2008.
About 95% of microorganisms cells dry-weight is composed of the major elements like carbon, oxygen, hydrogen, nitrogen, sulfur, phosphorus, potassium, calcium, magnesium and iron. They are required in large amounts and hence called as macroelemnts or macronutrients.Potassium is required for the activity of many enzymes and Calcium contribute to the heat resistance of the endospores. Magnesium serves as the cofactor for enzymes and complexes with ATP. Iron forms cofactor of enzymes and it is apart of the cytochromes. Sulphur is an essential compound of some amino acids and of the sulphur containing vitamin ,biotin .
Most microorganisms require manganese, zinc, cobalt,molybdenum, nickel and copper in very low amounts. Hence these are termed as trace elements or microelements.
Besides the common macroelements and trace elements ,microorganisms require particular substances which reflects their special nature of morphology or the surrounding environment.eg;Diatoms require silicic acid(H4SiO4) to construct their beautiful cell walls of silica.Although bacteria growing in saline lakes and oceans does not require large amounts of sodium because of the higher concentrations o sodium ion.
Image taken by Radheyshyam N Bhagat UCOL National Diploma in Science 2008.
Image taken by Radheyshyam N Bhagat PITO 2010.
Media:Nutritional_requirements.mp3
1.2
This description outlines how microorganisms can be subdivided into metabolic catagories depending on electron source and carbon source.
Many of the microorganisms can fix CO2 ie; reduce the CO2 and incorporate it into organic molecules.As carbon is required for the backbone of all organic molecules ,the requirements of macroelements like oxygen,hydrogen and carbon are satisfied whole together in microorganisms. Micro organisms are classified into two main subgroups, which are determined by the source of carbon. These two subgroups are autotrophs and heterotrophs.
Autotrophs use inorganic CO2 as their carbon source.
Heterotrophs use organic carbon sources such as sugars, proteins, fats or amino acids as their carbon source.
Micro organisms also require energy which in most cases is obtained through electron transfer, from electron donor to electron acceptor. Biological pathways are used for this electron transfer process, through which ATP can be formed.
There are three main sources from which micro organisms gain energy. These are light, and inorganic and organic compounds. Through these different methods of electron transfer, micro organisms can be further classified in to even smaller sub groups. These are phototrophs and chemotrophs.
Phototrophs use light, and the process of photosynthesis to produce energy.
Chemotrophs use organic and inorganic chemicals to produce energy.
Organotrophs are microorganisms that get their electron and hydrogen source from organic compounds.
Lithotrophs are microorganisms that their electron source from reduced inorganic compounds.
Photolithotrophs are microorganisms that get their energy source from light and their electron source from inorganic compounds.
Chemolithotrophs
These are microorganisms that get their energy from a chemical source and the electron source comes from inorganic compounds. Chemolithotrops are the organisms that obtain energy by oxidation of inorganic compounds. They are lithotropic in nature and thus they fix carbon dioxide .some of them are heterotrophic in nature. most of them uses Calvin cycle to fix carbon dioxide. To fix 1 mol of carbon dioxide by this pathway, 3 mol of ATP and 2 mol of NADPH+ H+ are required. This is obtained from oxidation of in organic molecules. A less amount of energy is available from this oxidation than from the oxidation of glucose to carbon dioxide. Electron cannot be donated directly from production of NADPH+ H+ to overcome this. ATP is required to drive the revised electron transport for the production of NADPH+ + H+.
Chemolithotrophs contribute greatly to the chemical transformations of elements (eg;the conversion of ammonia to nitrate and sulfur to sulfate which are normally occurring in the ecosystem).
Chemoorganotrophs
Chemoorganotrophs are often known as "Chemoheterotrophs", use organic compounds as a source of energy ,hydrogen,electrons and carbon for biosynthesis.The same organic nutrient will satisfy all these requirements.It should be noted that essential all pathogenic microorganisms are chemoheterotrophs.Some purple and green bacteria are photosynthetic and use organic matter as their electron donor and carbon source.
Photoorganotrops
Photoorganotrophs are known as "Photoorganoheterotrophs" , are common inhabitants of lakes and streams. Some of these bacteria can also grow as photoautotrophs with molecular hydrogen as an electron donor.
Mixotrophs
A few of the chemolithotrophs can derive their carbon from inorganic sources and thus are heterotrophic.Bacteria relying on inorganic energy sources and organic sources are called as Mixotrophs hence they are combining autotrophic and heterotrophic metabolic processes.
Prototrophs
A species or genetic strain of microbe capable of growing on a minimal medium consisting a simple carbohydrate or carbondioxide carbon source , with inorganic source of all other nutrient requirements.
Auxotrophs
A species or genetic strain requiring one or more complex organic nutrients ( such as amino acids , nucleotide bases , or enzymatic cofactors ) for growth.
2.1
The description outlines the oxidisation of glucose to pyruvate with the production of substrate level phosphorylation.
"Substrate Level Phosphorylation" is a process in which the phosphate group of a chemical compound is removed and directly added to ADP.,forming ATP,which contains the high energy phosphate bond.In cells it occurs primarily in the cytoplasm(in glycolysis)under both aerobic and anaerobic conditions.
To see production of substrate level phosphorilation, follow this link[2]
When microorganisms break down glucose, 2-phosphoglyceric acid is produced as a result. The atoms in this compound are then rearanged and a water molecule (H2O) is lost creating phosphoenolpyruvic acid which contains the high energy phosphate bond. ADP on the phosphate group of phosphoenolpyruvic acid has now turned into ATP. Follow this link to see diagramatical animation of glycolysis : http://www.youtube.com/watch?v=x-stLxqPt6E
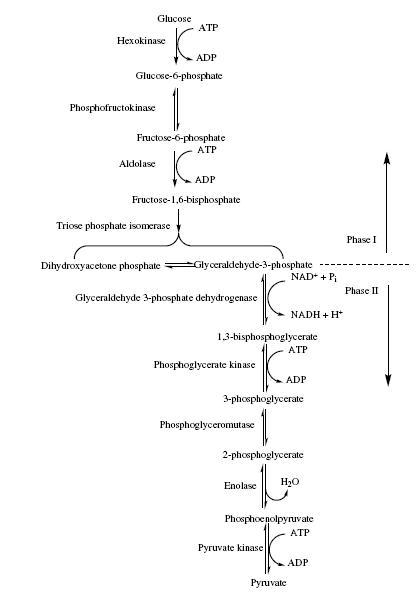
THE GLYCOLYTIC PATHWAY
It is the breakdown of glucose in to pyruvate and it is called as Embden-Meyerhof pathway also.In the initial six carbon stage glucose is phosphorylated twice and converted in to fructose1,6 bisphosphate with the expenditure of two ATP.Hence this preliminary step does not yield energy and it adds phosphate to each end of the sugar.
In the three carbon stage which is the second part of glycolysis starts when fructose 1,6 bisphosphate is cleaved by the enzyme fructose 1,6 bisphosphate aldolase into two halves, each with a phosphate group.One of the products,glyceraldehyde3-phosphate is converted directly in to pyruvate in a five step preocess.And the other product dihydroxyacetone phosphate can be changed to glyceraldehyde3-phosphate,and both the halves of fructose 1,6 bisphosphate are used in three carbon stage.Glyceraldehyde3-phosphate is first oxidised with NAD+ as the electron acceptor and a phosphate is incorporated to give a high energy molecule called 1,3 bisphosphoglycerate.Then the high enegry phosphate on carbon one is donated to ADP to produce ATP.This synthesis of ATP is calle as subsrate level phosphorylation as ADP phosphorylation is coupled with the breakdown of high energy substrate molecule.
A similar process produces another ATP by substrate level phosphorylation. When the phospahte group on the 3-phosphoglycerate shifts to carbon two, and the 2-phosphoglycerate is dehydrated to form a second high energy molecule called phosphoenol pyruvate.This phosphoenol pyruvate donates its phosphate to ADP to form second ATP and forms pyruvate which is the final product. For each glyceraldehyde3-phosphate transformed into pyruvate.one NADH and two ATPs are formed.Because two glyceraldehyde3-phosphate arise from the cleavage of fructose1,6 bisphosphate the second step in glycolysis generates total of 2 NADHs and 4 ATPs. Hence by substracting the 2 ATPs used in the first six carbon stage a total of 2 ATPs are formed.Thus the end products of glycolysis and substrate level phosphorylation can be concluded as:
Glucose + 2ADP + 2Pi(inorganic phosphur) + 2NAD+ ========================2 pyruvate + 2ATP + 2 NADH + 2 H+
GLUCOSE ->-> (intermediate reaction) ->->2 PHOSPHOGLYCERIN ACID (phosphate group)->->2 PHOSPHOGLYCERIN ACID (removal of water molecules) ->->HIGH ENERGY PHOSPHATE BOND->-> PHOSPHOENOL PYRUVIC ACID (ADENINE- RIBOSE (ADP)—P—P) ->-> SUBSTRATE LEVEL PHOSPHORYLATION->-> ADENINE--RIBOSE (ATP)-P-P~P (break of P) ->->-> PYRUVIC ACID
Fates of Pyruvate
1. Pyruvate to Acetyl CoA Pyruvic acid and NADH formed anerobically in the cytoplasm can release their energy only in aerobic conditions and in the presence of enzymes present inside.This reaction takes place with in mitochondrial matrix.
2. Pyruvate to lactate
Pyruvate + NADH --------------------------- Lactate + NAD
Lactate is produced under hypoxic condition in tissues e.g. skeletal muscle , renal medulla , retina , brain and GIT.
2.2
This outlines the oxidisation of pyruvate to carbon dioxide and production of ATP and equivalents by the tricarboxylic acid (TCA) cycle.
Trycarboxylic Acid(TCA)cycle is also known as "Citric Acid cycle" and "Kreb Cycle", is a series of enzyme catalysed chemical reactions of central importance in all living cells that uses oxygen as a part of cellular respiration.
Follow this link to see an animation of the citric acid cycle. [3] Follow this link to see the another diagramatical animation of Kreb Cycle or Citric Acid Cycle [4] The greater yield of energy is achieved in presence of oxygen from oxidation of pyruvate to carbon dioxide via TCA cycle. Before entering to this pathway it is converted acetyl COA and reaction is catalysed by pyruvate dehydrogenase.
(Pyruvate + NAD+ + COA -> Acetyl COA + NADH+ H+)
The citric acid cycle begins with acetyl-CoA transferring its two-carbon acetyl group to the four-carbon acceptor compound (oxaloacetate) to form a six-carbon compound (citrate). The citrate then goes through a series of chemical transformations, losing first one, then a second carboxyl group as CO2. The carbons lost as CO2 originate from what was oxaloacetate, not directly from acetyl-CoA. The carbons donated by acetyl-CoA become part of the oxaloacetate carbon backbone after the first turn of the citric acid cycle. Loss of the acetyl-CoA-donated carbons as CO2 requires several turns of the citric acid cycle. However, because of the role of the citric acid cycle in anabolism, they may not be lost since many TCA cycle intermediates are also used as precursors for the biosynthesis of other molecules.[5] Most of the energy made available by the oxidative steps of the cycle is transferred as energy-rich electrons to NAD+, forming NADH. For each acetyl group that enters the citric acid cycle, three molecules of NADH are produced. Electrons are also transferred to the electron acceptor Q, forming QH2. At the end of each cycle, the four-carbon oxaloacetate has been regenerated, and the cycle continues.
Kreb Cycle takes place in mitchondrial matrix . It is final common pathway for all food particles.
Importance of TCA cycle: 1. TCA cycle is amphibolic in nature. 2. Take part in gluconeogenesis transamination and deamination. 3. TCA take part in fatty acid synthesis.
ATP formed in one kreb's cycle : 3NADH + 1 NADH + ATP = 12 ATP
[edit] Products
2.3
This outlines the function of the electron transport chain to generate a proton-motive force to synthesise ATP. Electron Transport Chain Electron Transport System (ETS) is a series of coenzymes and cytochromes that take part in the transfer of electrons from a series of electron carrier molecules to its ultimate accepter.Electron during glucose oxidation enter the electron transport chain through NADH+ H+ OR FADH2. These electron are transported via number of carriers like pyridine, nucleotides, proteins, iron, cytochromes etc. Some of them are proton carrier with progressively more redox potential and reaches the oxygen. The energy liberated during this process is partially converted in synthesis of ATP.
Follow this link to see how the electron transport chain is used in conjunction with the enzyme ATP synthase, to create a proton motive force which in turn is responsible for the synthesis of ATP[[Media:[5]
Follow this link to see an animation on the electron transport chain. [6] Follow this link to see the diagrammatical explanation of Electron Transport http://www.youtube.com/watch?v=2IGIyA57Brc
In 1961 Peter Mitchell, a British biochemist, proposed chemiosmotic hypothesis which states that the electron transport chain is organized so that the protons move outward drom the mitochondrial matrix and electrons are transported inwards. The proton movement or the special proton pumps that derive the energy from the electron transport, resulting in generating the Proton motive force(PMF. PMF is a gradient of protons and a membrane potential due to the unequal distribution of charges.When these protons return to the mitichondrial matrix driven by the proton motive force,ATP is synthesized in a reversal of the ATP hydrolysis reaction.Hence in microorganisms also ATP synthesis occurs when these protons diffuse back in to the cell,when the electron flow causing the protons move outward across the plasma membrane.There are some exceptional cases like halophilic marine bacteria in which sodium ions may be used to drive ATP synthesis.
But many biologists believe in the conformational change hypothesis,in which the energy released by electron transport induces changes in the shape or conformation of the enzyme that synthesizes ATP. These conformational changes drives ATP formation, normally by changing the strength of ATP, ADP and phosphate binding to the enzymes catalytic site.
However, whatever is the real mechanism, it is certain that the ATP synthesis takes place at the F1F0 ATPase or ATP synthase; where F1 is the large complex with 3 alpha subunits and 2 beta subunits and F0 is the smaller complex with 3 subunits a,b,c respectively. The F0 complex participates in the proton movement across the membrane and this proton movement through a minute channel called as transmembrane proton channel.http://www.youtube.com/watch?v=s7A61fviPPM
2.4
The description outlines the nature and of terminal electron acceptors in aerobic and anaerobic respiration or outlines the production of ATP in fermentation.
Aerobic Respiration: "Aerobic Respiraion" , is the process in which there is release of energy from glucose in the existence of molecular oxygen.It occurs insides the mitochondria in most of plants and animals,called "Aerobes". It is divided into four phases: (1)Glycolysis (2)Oxidative Decarboxylation (3)Kreb Cycle or Citric Acid Cycle or TCA Cycle(4)Electron Transport System(ETC).
Follow this link to see the complete cellular respiration http://www.youtube.com/watch?v=AdtAu5JgOV0
To see ATP production in an aerobic environment follow this link.[7]
Anaerobic Respiration
Anaerobic respirationis the process of generating energy through the donation of electrons generated by oxidation of a nutrient to an external electron acceptor(other than oxygen). Anaerobic respiration is often incorrectly used inchangeably with fermentation. Fermantation occurs when an intracellular molecule generated by oxidation of glucose or another nutrient is the final electron acceptor. Anearobic is far less efficient than aerobic respiration, but many organisms can use it when necessary, usually when oxygen is lacking and they are called faculative anerobes.
Fermentation :Fermentation is a process in which organic molecules serve both as electron donors and accepters.In this process NADH is oxidised to NAD+.The electron accepter is either pyruvate or a pyruvate derivative. Follow this link to see how ATP is created by fermentation.[8]
Many fungi and some bacteria, algae and protozoa ferment sugars to ethanol and CO2 in a process called alcoholic fermentation.The reduction of pyruvate to lactate is called as Lactitc acid fermentation which has two types.One is the Homolatcic fermenters which uses the glycolytic pathway and directly reduce all of the pyruvate to lactate with the enzyme called as LDH or actate dehydrogenase.The second one is the Heterolactic fermenters form substantial amounts of products other than lactate like ethanol,CO2 by using phosphoketolase pathway.Enterobacteriaceae bacteria family can convert pyruvate to formic acid.It has two types ,one is Mixed acid fermentation resulting in excertion of ethanol amd a complex mixtyer of acetic,lactic succinic,and formic acids.The second type is Butanediol fermentation which metabolizes pyruvate to acetoin and which is then reduced to 2,3-butanediol with NADH along with large amount of ethanol.
Production of ATP in Fermentation
The major product of catabolic pathways is NADH+ H+. The is NADH+ H+ and FADH2 formed in TCA cycle, NADH+ H+ produced during glycolysis can be oxidized by electron transport pathway. However in absence oxygen, they oxidized back to NAD+. This leads to increase in ATP synthesis and which is important factor for organisms growing in absence of oxygen.
3.1-3.2
Idexx laboratories have developed a rapid detection method which utilizes the enzymatic process of target bacteria, to be positively identified and accurately enumerated.
This method is specifically used in conjunction with colilert substrate, for the detection of total coliforms and E. coli bacteria.
Colilert substrate is added to a 100ml sample which is then placed in a quanti tray (the quanti trays are shown in the photos below).

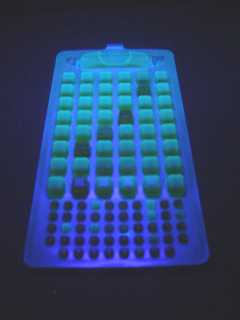
Photographs by D Beasley,NZ.
The quanti tray is then put through a machine that seals equal amounts of sample in each pouch, and is then incubated at 35oC for twenty four hours. All pouches in the quanti tray containing total coliforms turn yellow, and when placed under a UV light all pouches containing E. coli floures.
Within the colilert substrate are two nutrient indicators, ONPG (Ortho-nitrophenyl-b-D- galactopyranoside ) and MUG (4-methylumbelliferyl-β-D-glucuronide). These are the main carbon sources within the colilert substrate, which can be metabolized by coliform and E. coli enzymes, β-galactosidase and β-glucuronidase, respectively.
Any coliforms growing in the quanti trays turn the sample from colouless to yellow, by way of the enzymatic process of β-galactosidase metabolizing the ONPG. In a similar way E. coli uses the β-glucuronidase enzyme to metabolize MUG, which causes all pouches in the quanti tray containing E. coli, to floures when placed under UV light.
Most non coliforms have no effect on this test as they do not have β-galactosidase or β-glucuronidase enzymes, so cannot grow.
Enzyme activity--Nitrate reduction
Nitrate in organic nitrogen easily available for microbial use. The assimilation of nitrogen from nitrate into protein and cellular molecules called as assimilation nitrate reduction. In initial reaction nitrate is reduce to nitrite by enzyme nitrate reductase and nitrite then further reduced by a second enzyme nitrite reductase and finally reduced to ammonia. This happens through the involving the addition electron through series of reactions.
Urease Test
Amides are nitrogen containing organic compounds. An enzyme called Urease can create carbon dioxide, ammonia, and water by separating the carbon-nitrogen bond in amides (nitrogen containing organic compounds). If bacteria are inoculated onto a medium containing Urea, (an amide) you will be able to tell if the bacteria have the enzyme urease. If you insert pH indicator paper into the sample and it turns pink then urease is present if the indicator does not turn pink then urease is not present.
Ammonia is released when the urea is broken down hence raising the pH value.
Oxidase Test
The oxidase test is used to determine if an organism posseses the cytochrome oxidase enzyme. Oxidase positive bacteria posses cytochrome oxidase or indophenol oxidase ( an iron containing haemoprotein ). These both catalyse the transport of electrons from donor compounds ( NADH ) to electron acceptor ( usually oxygen ). The test reagent N,N,N,N-tetra-methyl-phenylenediamine dihydrochloride acts as an artificial electron acceptor for the enzyme oxidase. The oxidised reagent forms the coloured compound indophenol blue. The cytochrome system is usually onky present in aerobic organisms which are capable of utilising oxygen as the final hydrogen receptor. The end product of this metabolism is either water or hydrogen peroxide(broken down by catalyst)
Catalase Test
Toxic hydrogen peroxide is formed by some cytochromes. Catalase (an enzyme) breaksdown H2O2 (hydrogen peroxide) to O2 (free oxygen) and H2O (water).
If you were to grow bacteria on plates containing trypticase soy agar for example S.aureus and S.lactis by performing a simple biochemical test you can find out if the bacteria is catalase positive or catalase negative.
To determine this simply apply a few drops of 3% hydrogen peroxide to the different samples. If the sample starts to bubble this indicates a catalase positive test because oxygen is released as the catalase enzyme breaks down the hydrogen peroxide. Catalase negative test is represented by no bubbling. S.aures will bubble as this is catalase positive and S. lactis will not bubble as this is catalase negative.
Catabolic activity
Glycogen is formed and stored in liver and muscles. This is also found in human 'vagina' after puberty. Vaginal bacteria such as lactobacillus degrade this polymer into acidic. The end products that contribute to acidic pH of vagina. The glycogen storage found in bacteria such as Escherichia coli serves as food reserves for these microbial organisms. Bacteria uses endogenous glycogen as a carbon and energy by phosphorylysis and do not require expenditure of energy.
4.1-5.1
All life forms use ATP as a currency for energy.
Bacteria which are prokaryotes have no mitochondria for the manufacture or conversion of ATP, so use the cell membrane for this purpose.
As well as taking care of the bacterial cells energy needs, the cell membrane is also responsible for the synthesis of structural macromolecules, nutrient processing, and enzyme secretion all requirements of the bacterial cell.
Bacteria use the enzyme ATP synthase in conjunction with the electron transfer chain to convert ADP+Pi to ATP, a process which takes place in the cell membrane between the phospholipid bilayer in bacteria.
This is a simple process that requires a relatively complex biological mechanism and can be better explained and understood by following this link[9], and watching the animation.
Chemolithotrophy These are organisms which obtains energy by oxidizing inorganic chemical compounds, energy is liberated as they remove electron from the molecules of theses compounds.Most of the species are usually autotrophic and use carbon dioxide(CO2)as their major source of energy.These are mostly occur in soil and water.The best suited nitrogen chemolithotrophs are the nitrifying bacteria.These are nonpathogenic in nature.
The energy molecule ATP is formed as result of electron chain activity in both the aerobic and anaerobic repsiration.Electrons from the chain can be obtained from the oxidation of inorganic molecules rather than from organic nutrients.A small group of bacteria falls in to this confined group are called Chemolithotrophs.Each species is specifc in its preferences for electron donors and and acceptors.The electron acceptor is usually O2 but sulfates and nitrates are used in some cases. Chemolithotrophic bacteria are normally autotrophic and employ Calvin cycle to fix CO2 as their carbon source.Many chemolithotrophs can oxidize hydrogen gas to produce energybecause they possses a hydrogenase enzyme that catalyses the oxidation of hydrogen.Here the electrons are donated to either to an electron transport chain or to NAD+ depending on the hydrogenase enzyme.If NADH is produced,it can be used in electron transport and oxidative phosphorylation ,with O2 as the terminal electron acceptor. As in case of the Nitrifying bacteria Nitrosomanas oxidizes ammonia to nitrite andthis nitrite can be oxidized by Nitrobacter to yield nitrate.The combined work of these two microbes converts the ammonia in the soil to nitrate and is called as nitrification.
Phototrophy
These are the organisms which uses the light as for the primary source of energy. They use the radiant energy from the sun and convert into the chemical energy from the sun and convert into the chemical energy in the form of carbohydrate and other molecules.The process in which light energy is trapped and converted in to chemical energy in the form of ATP,NADH or NADPH is called as photosynthesis and over half of which is carried out on earth by microbes.
Phototrophs possess light absorbing pigments called chlorophyll a and chlorophyll b and carotenoids and phycobibliprotiens which are assembled in highly organized arrays called as antennas having around 300 molecules of chlorophylls. Photosystem I absorbs longer wavelength >680nm and funnels the energy to a special chlorophyll a molecule called P700 which absorbs light at wavelength of 700nm.Photosystem II traps light at a wavelenght <680nm and tranfers its energy to other pigment chlorophyll P680. Chlorophyll a has a light absorption peak at 665nm and chlorophyll b has 645nm.Because cholorophyll mostly absorbs light at the red and blue regions in the electromagnetic radiation spectrum , the green light is being transmitted,hence the photosynthetic microbes are green in colour.Red algae and cyanobacterium have pigments called phycobiliprotiens cosisting of protien with tetrapyrrole attached to it. It has Phycoerythrin red pigment with maximum absorption at 550nm and Phycocyanin blue pigment with 620-640 nm maximum absorption.
5.1
The discussion outlines the process of ion and molecular transport across the cell membrane
The cytoplasmic membrane decides what enters and exits a cell.
Passive Transport means moving biochemicals and atomic or molecular substances across the cell membrane. unlike active transport, this process involve chemical energy. the four main kinds of passive transport are Diffusion, Facilitated diffusion, Filtration and Osmosis.
Simple diffusion is where nutrient molecules can freely enter and exit a cell through the cytoplasmic membrane. Molecules from outside the cell will move inwards until the concentration is equal both in side and outside the cell. The concentration of the nutrient molecules will not be higher inside the cell then out. The molecule move from high concentration to lower concentration. More of water in side the cell than out side and the carbon dioxide moves out by simple diffusion. Metabolic energy is not needed for simple diffusion.
Facilitated diffusion is similar to simple diffusion in the fact that is does not require metabolic energy and the nutrient molecule concentration is the same inside and outside the cell. The difference is that in order for the nutrient molecules to pass through the cytoplasmic membrane they must first attach themselves to a carrier protein.
Active transport is the way in which the vast majority of nutrients enter a cell. The nutrient molecules enter the cell attached to a carrier protein like they do in facilitated diffusion. The difference between active transport and facilitated diffusion is that active transport requires metabolic energy to help push the nutrient molecules across the cytoplasmic membrane. The energy is needed because the concentration of nutrient molecules can be hundreds of times higher inside the cell than outside. Group Translocationis a process in which a molecule is a transported into the cell while being chemically changed.In this process procaryotes also take up molecules.The best-known translocation is phosphoenolpyruvate:sugarphosphotransferase system (PTS) .It transports different types of sugars into procaryotic cells while phosphorlayting them using phosphoenolpyruvate (PEP)as a phosphate donor. PEP + sugar -----> pyruvate + sugar. The PTS is quite complex.
Exocytosis is the process in which the membrane bound vesicle in the cytoplasm fuses with the plasma membrane, to move the things from inside to the outside the cell. This material accumulates in the plasma membrane and this way parts of the plasma membrane lost during endocytosis is replaced. This process provides the routes to membranes impermeable molecules such as proteins hormones that are synthesized by the cell which can be released into the extracellualr materials. This process is use to continue the increase in cystolic calcium concentration which in turn activates proteins essential for vesicle membrane to fuse with the plasma membrane and continuing the process to maintain the holes from the prior process. http://www.youtube.com/watch?v=U9pvm_4-bHg
References
1.Bacteria. (2008). In Encyclopædia Britannica. Retrieved September 22, 2008, from Encyclopædia Britannica Online: [10]
2.Welcome to BIOL 230 Lecture on the Web. (2008). Retrieved October 2, 2008, from [11]
3.Idexx labratories. (2008). Retrieved October 30, 2008, from [http://www.idexx.com/aboutidexx/
]
4.The True Origin Archive. (2008). ATP: The Perfect Energy Currency for the Cell, Jerry Bergman,Ph.D. Retrieved October 30, 2008, from [12]
5.Alcomo's Fundamentals of Microbilogy
6.Microbiology by Michael J. Pelczar, E.C.S Chan, Noel R.Krieg.
7.Microbiology by LANSING M.PRESCOTT,JOHN P.HARLEY,DONALD A.KLEIN
8.Microbiology concepts and application by Chan, Kreig, Pelczar