Lab: Cell respiration
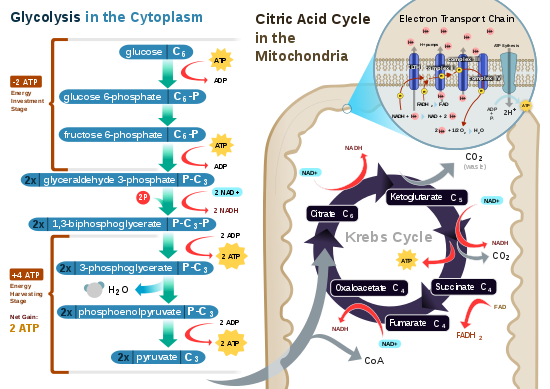
| Cellular respiration is the set of the metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products. The reactions involved in respiration are catabolic reactions that involve the redox reaction (oxidation of one molecule and the reduction of another). Respiration is one of the key ways a cell gains useful energy to fuel cellular reformations.
Aerobic respiration requires oxygen in order to generate energy (ATP). The equation below shows the complete oxidation of glucose.
yielding a maximum of 38 ATP molecules per oxidised glucose molecule, although due to certain inefficiencies the yield is estimated at between 29 to 30 ATP per glucose. This extract is licensed under the Creative Commons Attribution-ShareAlike license. It uses material from the article "Cell respiration", retrieved 27 Mar 2011. |
This lab offers an opportunity for students to observe evidence for respiration in seeds. A seed is a living organism. It is a small embryonic plant, enclosed in a covering called the seed coat, usually along with some stored food. A seed is considered dormant until the necessary conditions for germination are met. Germination "involves the reactivation of the metabolic pathways that lead to growth."[1] As a seed moves into germination the rate of cellular respiration greatly increases.[2]
There are a few possible ways that the results of respiration might be observed. Look back at the glucose oxidation equation to consider how we might measure respiration. Compare your ideas to those provided below (click the down arrow on the right to show the options):
We could measure....
|
In this lab you will compare the relative volume of oxygen consumed by germinating and non-germinating (dry) pea seeds and investigate the effect of temperature on the rate of consumption (one of a number of factors that could affect the respiration process). Respirometers will be used to measure the change in gas volume for germinating and non-germinating pea seeds at two different temperatures.[3][4]
Contents
Prep
In preparation for the experiment:
- Review cell respiration in a textbook, using the The Biology Place BioCoach or using another resource.
- Review the basics of how this experiment works at Cell Respiration, one of the labs at The Biology Place Lab Bench.
Additional background
The ideal gas law is fundamental to the understanding of how the respirometer works. The state of an amount of gas is determined by its pressure, volume, and temperature. The modern form of the equation is:
- [math]pV = nRT\,[/math]
where
- p is the absolute pressure of the gas;
- V is the Volume of the gas;
- n is the number of molecules of gas;
- R is the ideal, or universal, gas constant; and
- T is the absolute temperature of the gas (in Ko).[5]
The following principles[4] follow from relationship denoted in the ideal gas law:
- If temperature and pressure are constant, then the volume of the gas is directly proportional to the number of molecules of gas.
- If the temperature and volume are constant, then the pressure of the gas is in direct proportion to the number of molecules of gas present.
- If the number of gas molecules and the temperature are constant, then the pressure is inversely proportional to the volume.
- If the temperature changes and the number of gas molecules are constant, then either pressure or volume (or both) will change in direct proportion to the temperature.
In this lab, we will use sodium hydroxide (NaOH) as an agent to combine with the CO2, produced as a result of cellular respiration, to form solid sodium carbonate (Na2CO3) according to the following reaction:
- CO2 + 2 NaOH → Na2CO3 + H2O
Question: Knowing that the O2 gas is consumed during cellular respiration, and if the CO2 gas is removed, which of the above principles related to the ideal gas law will apply to the gas in the respirometer? (Click the arrow below on the right for an explanation.)
The relevant principle is
|
A respirometer will be set-up for each of the experimental conditions: 3 sources of respiration (germinating pea, dry pea, plastic bead) and 2 temperatures (room temp and 10oC. Study the diagram of the respirometer setup at BioTopics, The Respiration Process. (Scroll down to near the bottom, "a simple respirometer".)
Printout the materials and procedure for reference during the lab.
Materials Needed
- Respirometers
- Masking tape
- Sharpie
- Peas, half germinating and half dry
- Beads (or other small non-floating objects)
- Ice
- 2 Containers for water baths
- Thermometer
- Food coloring, 1-2 dark colors
- Cotton balls
- KOH (potassium hydroxide) or NaOH (sodium hydroxide)*
- Vaseline (or putty or non-hardening clay....used to seal the respirometers)
- *Potassium hydroxide and sodium hydroxide are harmful if brought into contact with the eye, skin or if swallowed. Goggles should be worn when using these solutions. See links for further safety information:
Procedure
Before beginning the experiment, prepare a data table to record the results for the 6 experimental conditions.
Use the following procedure[4] as a guide in performing the experiment:
- Prepare a room-temperature bath (approx. 25oC) and a cold-water bath (approx. 10oC).
- Prepare two containers of dark-colored water.
- Prepare the contents of the respirometers which will go in the room temperature bath. Set the beads aside.
- Determine the number of germinating peas that fit into the 9-dram vial in the respirometer. Find the volume of these germinating peas, using water displacement.
- Measure the volume of the same number of dry peas and add beads to attain an equal volume to the germinating peas.
- Measure an amount of beads to the same volume as the germinating peas.
- Repeat the previous step to prepare contents for respirometers which will go in the 10oC bath.
- Number each of 6 respirometers (a 9-dram vial with tubing inserted and caulked through lid) using tape/sharpie.
- Place a small wad of absorbent cotton in the bottom of each vial and, using a pipette, saturate the cotton with 15% NaOH (sodium hydroxide). It is important that the same amount of NaOH be used for each respirometer.
- Place a small wad of dry, nonabsorbent material on top of the saturated cotton.
- Place the first set of germinating peas, dry peas and beads in vials 1-3, respectively.
- Place the next set of germinating peas, dry peas and beads in vials 4-6, respectively.
- Recap the vials. Seal the edge of the lid with vaseline or non-hardening clay
- Weight each vial by attaching something heavy to the outside.
- Place the vials in their respective baths for 7 minutes.
- After 7 min, put the free ends of the tubing into the beakers of colored water. A little water should enter the tubing and then stop. If the water continues to enter the tubing, check for leaks in the respirometer.
- Allow the respirometers to equilibrate for 3 more minutes and then mark the initial position of the water in each tube (time 0) with a piece of tape.
- Check the temperature in both baths and record.
- Every 5 minutes for 20 minutes, measure the water level (in centimeters from the starting position) in the six tubes. Record measurements in your data table.
Some ideas for results/conclusion/discussion
Use the following ideas[4] to further your understanding of the results:
- The beads are included as a control. How can the results for the beads be used to "correct" the results for the peas?
- Graph the results for the germinating peas and dry peas for each temperature.
- How does the amount of O2 consumed change over time in the different conditions.
- Determine the rate of O2 consumption (the slope of the line) of germinating and dry peas during the experiments at each temperature.
- What is the effect of germination on the respiration in peas?
- Why did the vial have to be completely sealed around the lid and around the tubing/lid connection?
Notes
- ↑ Seed. In Wikipedia, accessed 27 Mar 2011.
- ↑ Steane, Richard. The Respiration Process. BioTopics, accessed 27 Mar 2011.
- ↑ 3.0 3.1 Billingsley, J. and Miller, L. Cellular Respiration Lab, EDHS Green Sea, accessed 27 mar 2011.
- ↑ 4.0 4.1 4.2 4.3 Nuño, J. S. Laboratory 5: Cell Respiration, jdenuno.com, accessed 27 Mar 2011.
- ↑ Ideal gas law. In Wikipedia, accessed 27 Mar 2011.