How Solar Yard Lights Work
If you have a yard and have ever thought about lighting it at night, then you have probably heard about solar yard lights. They are still pretty expensive, but their advantage is that you don't have to run any wiring for them. As long as a location gets direct sunlight, you can put a light there in about 15 seconds.
These lights are extremely interesting because they are almost like mini-satellites. They generate and store their own power during the day and then release it at night. This is just like a satellite that stores solar energy while it is on the sunny side of the planet and then uses that energy when it's on the dark side. In this article, you will learn exactly how it happens!
Contents
[hide]The Basics
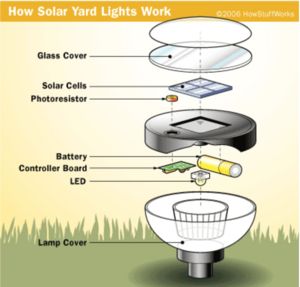
Here is a typical solar yard light:
Solar lights are an energy-efficient choice.
It consists of the following components:
- A plastic case
- A solar cell on top
- A single AA Nicad battery
- A small controller board
- An LED light source
- A photoresistor to detect darkness
If you pop off the cover, you will find that all of the working components are mounted as a single unit. On the back side you see this:
Next, we'll take a closer look at some of these components.
Inside a Solar Yard Light
You can see the battery, LED and controller board. If you cover the light sensor, the LED turns on, like this:
Here are close-ups of the LED and the controller board:
On the other side of this module is a four-cell solar array, measuring 2 inches by 2 inches (5 cm x 5 cm), and the photoresistor:
Here is a closer view of the photoresistor:
Producing Light
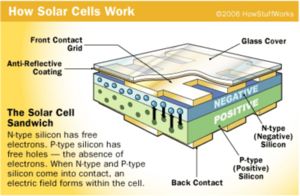
If you have read the article How Solar Cells Work, you have a basic understanding of solar-cell technology. A solar yard light uses standard solar cells in a very straightforward application.
A single solar cell produces a maximum of 0.45 volts and a varying amount of current depending on the size of the cell and the amount of light striking the surface. In a typical yard light, therefore, you need four cells wired in series (see How Batteries Work for a discussion on series wiring). In this yard light, the four cells will produce 1.8 volts and a maximum of about 100 milliamps in full, bright sunlight.
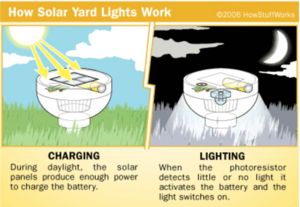
The solar cells are wired directly to the battery through a diode (which prevents the battery's current from flowing back through the solar cell at night). The battery is a completely standard AA Nicad battery. A battery like this produces about 1.2 volts and can store a maximum of approximately 700 milliamp-hours. During the day, the battery charges, reaching maximum charge except on shorter winter days or days when there is heavy overcast.
At night, the solar cells stop producing power. The photoresistor turns on the LED. How do the streetlights turn on automatically at night? shows you a very simple circuit using a transistor and a relay to control a light using a photoresistor. In the case of this light, the relay is replaced by two other transistors.
The controller board accepts power from the solar cell and battery, as well as input from the photoresistor. It has a three-transistor circuit that turns on the LED when the photoresistor indicates darkness.
The LED draws about 45 milliamps with the battery producing about 1.23 volts (0.055 watts). It produces about half of the light that a candle would. The Nicad battery, when fully charged, can operate the LED for about 15 hours.
Half of a candle's light is not very much, and if you have ever purchased one of these yard lights you know that it really is not enough to provide illumination. You use them more for marking a trail -- they are bright enough to see, but not really bright enough to illuminate the ground to any great degree.
The reason why these lights are so expensive right now is because of the solar cells and, to a lesser degree, the Nicad battery. Solar cells remain expensive because they are manufactured from silicon crystals in cleanroom conditions. They are much less expensive than they were 10 or 20 years ago, but still fairly pricey. As a result, solar yard lights cost $10 to $20 per light.
The yard light shown here uses a single LED. More expensive lights may offer a combination of an LED and a small halogen flashlight bulb. The LED is on all the time, and the light bulb turns on for a minute or two when a motion sensor detects movement.
We see things every day, from the moment we get up in the morning until we go to sleep at night. We look at everything around us using light. We appreciate kids' crayon drawings, fine oil paintings, swirling computer graphics, gorgeous sunsets, a blue sky, shooting stars and rainbows. We rely on mirrors to make ourselves presentable, and sparkling gemstones to show affection. But did you ever stop to think that when we see any of these things, we are not directly connected to it? We are, in fact, seeing light -- light that somehow left objects far or near and reached our eyes. Light is all our eyes can really see.
The other way that we encounter light is in devices that produce light -- incandescent bulbs, fluorescent bulbs, lasers, lightning bugs, the sun. Each one uses a different technique to generate photons.
In this article, we will look at light from many different angles to show you exactly how it works!
Ways of Thinking About Light
You have probably heard two different ways of talking about light:
- There is the "particle" theory, expressed in part by the word photon.
- There is the "wave" theory, expressed by the term light wave.
From the time of the ancient Greeks, people have thought of light as a stream of tiny particles. After all, light travels in straight lines and bounces off a mirror much like a ball bouncing off a wall. No one had actually seen particles of light, but even now it's easy to explain why that might be. The particles could be too small, or moving too fast, to be seen, or perhaps our eyes see right through them.
The idea of the light wave came from Christian Huygens, who proposed in the late 1600s that light acted like a wave instead of a stream of particles. In 1807, Thomas Young backed up Huygens' theory by showing that when light passes through a very narrow opening, it can spread out, and interfere with light passing through another opening. Young shined a light through a very narrow slit. What he saw was a bright bar of light that corresponded to the slit. But that was not all he saw. Young also perceived additional light, not as bright, in the areas around the bar. If light were a stream of particles, this additional light would not have been there. This experiment suggested that light spread out like a wave. In fact, a beam of light radiates outward at all times.
Albert Einstein advanced the theory of light further in 1905. Einstein considered the photoelectric effect, in which ultraviolet light hits a surface and causes electrons to be emitted from the surface. Einstein's explanation for this was that light was made up of a stream of energy packets called photons.
Modern physicists believe that light can behave as both a particle and a wave, but they also recognize that either view is a simple explanation for something more complex. In this article, we will talk about light as waves, because this provides the best explanation for most of the phenomena our eyes can see.
What is Light?
Why is it that a beam of light radiates outward, as Young proved? What is really going on? To understand light waves, it helps to start by discussing a more familiar kind of wave -- the one we see in the water. One key point to keep in mind about the water wave is that it is not made up of water: The wave is made up of energy traveling through the water. If a wave moves across a pool from left to right, this does not mean that the water on the left side of the pool is moving to the right side of the pool. The water has actually stayed about where it was. It is the wave that has moved. When you move your hand through a filled bathtub, you make a wave, because you are putting your energy into the water. The energy travels through the water in the form of the wave.
All waves are traveling energy, and they are usually moving through some medium, such as water. You can see a diagram of a water wave in Figure 1. A water wave consists of water molecules that vibrate up and down at right angles to the direction of motion of the wave. This type of wave is called a transverse wave.
Light waves are a little more complicated, and they do not need a medium to travel through. They can travel through a vacuum. A light wave consists of energy in the form of electric and magnetic fields. The fields vibrate at right angles to the direction of movement of the wave, and at right angles to each other. Because light has both electric and magnetic fields, it is also referred to as electromagnetic radiation.
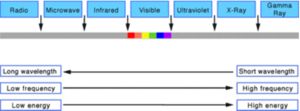
Light waves come in many sizes. The size of a wave is measured as its wavelength, which is the distance between any two corresponding points on successive waves, usually peak-to-peak or trough-to-trough (Figure 1). The wavelengths of the light we can see range from 400 to 700 billionths of a meter. But the full range of wavelengths included in the definition of electromagnetic radiation extends from one billionth of a meter, as in gamma rays, to centimeters and meters, as in radio waves. Light is one small part of the spectrum.
Frequencies
Light waves also come in many frequencies. The frequency is the number of waves that pass a point in space during any time interval, usually one second. It is measured in units of cycles (waves) per second, or Hertz (Hz). The frequency of visible light is referred to as color, and ranges from 430 trillion Hz, seen as red, to 750 trillion Hz, seen as violet. Again, the full range of frequencies extends beyond the visible spectrum, from less than one billion Hz, as in radio waves, to greater than 3 billion billion Hz, as in gamma rays.
As noted above, light waves are waves of energy. The amount of energy in a light wave is proportionally related to its frequency: High frequency light has high energy; low frequency light has low energy. Thus gamma rays have the most energy, and radio waves have the least. Of visible light, violet has the most energy and red the least.
Light not only vibrates at different frequencies, it also travels at different speeds. Light waves move through a vacuum at their maximum speed, 300,000 kilometers per second or 186,000 miles per second, which makes light the fastest phenomenon in the universe. Light waves slow down when they travel inside substances, such as air, water, glass or a diamond. The way different substances affect the speed at which light travels is key to understanding the bending of light, or refraction, which we will discuss later.
So light waves come in a continuous variety of sizes, frequencies and energies. We refer to this continuum as the electromagnetic spectrum (Figure 2). Figure 2 is not drawn to scale, in that visible light occupies only one-thousandth of a percent of the spectrum.
Producing a Photon
Any light that you see is made up of a collection of one or more photons propagating through space as electromagnetic waves. In total darkness, our eyes are actually able to sense single photons, but generally what we see in our daily lives comes to us in the form of zillions of photons produced by light sources and reflected off objects. If you look around you right now, there is probably a light source in the room producing photons, and objects in the room that reflect those photons. Your eyes absorb some of the photons flowing through the room, and that is how you see.
There are many different ways to produce photons, but all of them use the same mechanism inside an atom to do it. This mechanism involves the energizing of electrons orbiting each atom's nucleus. How Nuclear Radiation Works describes protons, neutrons and electrons in some detail. For example, hydrogen atoms have one electron orbiting the nucleus. Helium atoms have two electrons orbiting the nucleus. Aluminum atoms have 13 electrons orbiting the nucleus. Each atom has a preferred number of electrons orbiting its nucleus.
Electrons circle the nucleus in fixed orbits -- a simplified way to think about it is to imagine how satellites orbit the Earth. There's a huge amount of theory around electron orbitals, but to understand light there is just one key fact to understand: An electron has a natural orbit that it occupies, but if you energize an atom you can move its electrons to higher orbitals. A photon of light is produced whenever an electron in a higher-than-normal orbit falls back to its normal orbit. During the fall from high-energy to normal-energy, the electron emits a photon -- a packet of energy -- with very specific characteristics. The photon has a frequency, or color, that exactly matches the distance the electron falls.
There are cases where you can see this phenomenon quite clearly. For example, in lots of factories and parking lots you see sodium vapor lights. You can tell a sodium vapor light because it is very yellow when you look at it. A sodium vapor light energizes sodium atoms to generate photons. A sodium atom has 11 electrons, and because of the way they are stacked in orbitals one of those electrons is most likely to accept and emit energy (this electron is called the 3s electron, and is explained on this page). The energy packets that this electron is most likely to emit fall right around a wavelength of 590 nanometers. This wavelength corresponds to yellow light. If you run sodium light through a prism, you do not see a rainbow -- you see a pair of yellow lines.
Bring on the Heat
Probably the most common way to energize atoms is with heat, and this is the basis of incandescence. If you heat up a horseshoe with a blowtorch, it will eventually get red hot, and if you heat it enough it gets white hot. Red is the lowest-energy visible light, so in a red-hot object the atoms are just getting enough energy to begin emitting light that we can see. Once you apply enough heat to cause white light, you are energizing so many different electrons in so many different ways that all of the colors are being generated -- they all mix together to look white, as explained in one of the sections below.
Heat is the most common way we see light being generated -- a normal 75-watt incandescent bulb is generating light by using electricity to create heat. However, there are lots of other ways to generate light, some of which are listed below:
- Halogen lamps - Halogen lamps use electricity to generate heat, but benefit from a technique that lets the filament run hotter.
- Gas lanterns - A gas lantern uses a fuel like natural gas or kerosene as the source of heat.
- Fluorescent lights - Fluorescent lights use electricity to directly energize atoms rather than requiring heat.
- Lasers - Lasers use energy to "pump" a lasing medium, and all of the energized atoms are made to dump their energy at the exact same wavelength and phase.
- Glow-in-the-dark toys - In a glow-in-the-dark toy, the electrons are energized but fall back to lower-energy orbitals over a long period of time, so the toy can glow for half an hour.
- Indiglo watches - In Indiglo watches, voltage energizes phosphor atoms.
- Chemical light sticks - A chemical light stick and, for that matter, fireflies, use a chemical reaction to energize atoms.
The thing to note from this list is that anything that produces light does it by energizing atoms in some way.
Let's start with a normal electric light bulb like you see in any normal household lamp. A normal light bulb is made up of a fairly large, thin, frosted glass envelope. Inside the glass is a gas such as argon and/or nitrogen. At the center of the lamp is a tungsten filament. Electricity heats this filament up to about 4,500 degrees F (2,500 degrees Celsius). Just like any hot metal, the tungsten gets "white hot" at that heat and emits a great deal of visible light in a process called incandescence. See How Gas Lanterns Work for more information on incandescence.
A normal light bulb is not very efficient, and it only lasts about 750 to 1,000 hours in normal use. It's not very efficient because, in the process of radiating light, it also radiates a huge amount of infrared heat -- far more heat than light. Since the purpose of a light bulb is to generate light, the heat is wasted energy. It doesn't last very long because the tungsten in the filament evaporates and deposits on the glass. Eventually, a thin spot in the filament causes the filament to break, and the bulb "burns out."
A halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. Because the envelope is so close to the filament, it would melt if it were made from glass. The gas inside the envelope is also different -- it consists of a gas from the halogen group. These gases have a very interesting property: They combine with tungsten vapor. If the temperature is high enough, the halogen gas will combine with tungsten atoms as they evaporate and redeposit them on the filament. This recycling process lets the filament last a lot longer. In addition, it is now possible to run the filament hotter, meaning you get more light per unit of energy. You still get a lot of heat, though; and because the quartz envelope is so close to the filament, it is extremely hot compared to a normal light bulb.