How Solar Cells Work PHY 361 2008
by Scott Aldous
Contents
Introduction to How Solar Cells Work
You've probably seen calculators that have solar cells -- calculators that never need batteries, and in some cases don't even have an off button. As long as you have enough light, they seem to work forever. You may have seen larger solar panels -- on emergency road signs or call boxes, on buoys, even in parking lots to power lights. Although these larger panels aren't as common as solar powered calculators, they're out there, and not that hard to spot if you know where to look. There are solar cell arrays on satellites, where they are used to power the electrical systems.
You have probably also been hearing about the "solar revolution" for the last 20 years -- the idea that one day we will all use free electricity from the sun. This is a seductive promise: On a bright, sunny day, the sun shines approximately 1,000 watts of energy per square meter of the planet's surface, and if we could collect all of that energy we could easily power our homes and offices for free.
In this article, we will examine solar cells to learn how they convert the sun's energy directly into electricity. In the process, you will learn why we are getting closer to using the sun's energy on a daily basis, and why we still have more research to do before the process becomes cost effective.
Going Solar, Going Green
Adding solar panels to an existing home can be expensive -- but there are lots of other ways to make your home greener. Learn more about what you can do to protect the environment at Discovery Channel's Planet Green.
Photovoltaic Cells: Converting Photons to Electrons
The solar cells that you see on calculators and satellites are photovoltaic cells or modules (modules are simply a group of cells electrically connected and packaged in one frame). Photovoltaics, as the word implies (photo = light, voltaic = electricity), convert sunlight directly into electricity. Once used almost exclusively in space, photovoltaics are used more and more in less exotic ways. They could even power your house. How do these devices work?
Photovoltaic (PV) cells are made of special materials called semiconductors such as silicon, which is currently the most commonly used. Basically, when light strikes the cell, a certain portion of it is absorbed within the semiconductor material. This means that the energy of the absorbed light is transferred to the semiconductor. The energy knocks electrons loose, allowing them to flow freely. PV cells also all have one or more electric fields that act to force electrons freed by light absorption to flow in a certain direction. This flow of electrons is a current, and by placing metal contacts on the top and bottom of the PV cell, we can draw that current off to use externally. For example, the current can power a calculator. This current, together with the cell's voltage (which is a result of its built-in electric field or fields), defines the power (or wattage) that the solar cell can produce.
That's the basic process, but there's really much more to it. Let's take a deeper look into one example of a PV cell: the single-crystal silicon cell.
How Silicon Makes a Solar Cell
Silicon has some special chemical properties, especially in its crystalline form. An atom of silicon has 14 electrons, arranged in three different shells. The first two shells, those closest to the center, are completely full. The outer shell, however, is only half full, having only four electrons. A silicon atom will always look for ways to fill up its last shell (which would like to have eight electrons). To do this, it will share electrons with four of its neighbor silicon atoms. It's like every atom holds hands with its neighbors, except that in this case, each atom has four hands joined to four neighbors. That's what forms the crystalline structure, and that structure turns out to be important to this type of PV cell.
We've now described pure, crystalline silicon. Pure silicon is a poor conductor of electricity because none of its electrons are free to move about, as electrons are in good conductors such as copper. Instead, the electrons are all locked in the crystalline structure. The silicon in a solar cell is modified slightly so that it will work as a solar cell.
A solar cell has silicon with impurities -- other atoms mixed in with the silicon atoms, changing the way things work a bit. We usually think of impurities as something undesirable, but in our case, our cell wouldn't work without them. These impurities are actually put there on purpose. Consider silicon with an atom of phosphorous here and there, maybe one for every million silicon atoms. Phosphorous has five electrons in its outer shell, not four. It still bonds with its silicon neighbor atoms, but in a sense, the phosphorous has one electron that doesn't have anyone to hold hands with. It doesn't form part of a bond, but there is a positive proton in the phosphorous nucleus holding it in place.
When energy is added to pure silicon, for example in the form of heat, it can cause a few electrons to break free of their bonds and leave their atoms. A hole is left behind in each case. These electrons then wander randomly around the crystalline lattice looking for another hole to fall into. These electrons are called free carriers, and can carry electrical current. There are so few of them in pure silicon, however, that they aren't very useful. Our impure silicon with phosphorous atoms mixed in is a different story. It turns out that it takes a lot less energy to knock loose one of our "extra" phosphorous electrons because they aren't tied up in a bond -- their neighbors aren't holding them back. As a result, most of these electrons do break free, and we have a lot more free carriers than we would have in pure silicon. The process of adding impurities on purpose is called doping, and when doped with phosphorous, the resulting silicon is called N-type ("n" for negative) because of the prevalence of free electrons. N-type doped silicon is a much better conductor than pure silicon is.
Actually, only part of our solar cell is N-type. The other part is doped with boron, which has only three electrons in its outer shell instead of four, to become P-type silicon. Instead of having free electrons, P-type silicon ("p" for positive) has free holes. Holes really are just the absence of electrons, so they carry the opposite (positive) charge. They move around just like electrons do.
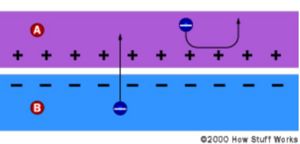
The interesting part starts when you put N-type silicon together with P-type silicon. Remember that every PV cell has at least one electric field. Without an electric field, the cell wouldn't work, and this field forms when the N-type and P-type silicon are in contact. Suddenly, the free electrons in the N side, which have been looking all over for holes to fall into, see all the free holes on the P side, and there's a mad rush to fill them in.
Anatomy of a Solar Cell
Before now, our silicon was all electrically neutral. Our extra electrons were balanced out by the extra protons in the phosphorous. Our missing electrons (holes) were balanced out by the missing protons in the boron. When the holes and electrons mix at the junction between N-type and P-type silicon, however, that neutrality is disrupted. Do all the free electrons fill all the free holes? No. If they did, then the whole arrangement wouldn't be very useful. Right at the junction, however, they do mix and form a barrier, making it harder and harder for electrons on the N side to cross to the P side. Eventually, equilibrium is reached, and we have an electric field separating the two sides.
This electric field acts as a diode, allowing (and even pushing) electrons to flow from the P side to the N side, but not the other way around. It's like a hill -- electrons can easily go down the hill (to the N side), but can't climb it (to the P side).
So we've got an electric field acting as a diode in which electrons can only move in one direction.
When light, in the form of photons, hits our solar cell, its energy frees electron-hole pairs.
Each photon with enough energy will normally free exactly one electron, and result in a free hole as well. If this happens close enough to the electric field, or if free electron and free hole happen to wander into its range of influence, the field will send the electron to the N side and the hole to the P side. This causes further disruption of electrical neutrality, and if we provide an external current path, electrons will flow through the path to their original side (the P side) to unite with holes that the electric field sent there, doing work for us along the way. The electron flow provides the current, and the cell's electric field causes a voltage. With both current and voltage, we have power, which is the product of the two.
There are a few more steps left before we can really use our cell. Silicon happens to be a very shiny material, which means that it is very reflective. Photons that are reflected can't be used by the cell. For that reason, an antireflective coating is applied to the top of the cell to reduce reflection losses to less than 5 percent.
The final step is the glass cover plate that protects the cell from the elements. PV modules are made by connecting several cells (usually 36) in series and parallel to achieve useful levels of voltage and current, and putting them in a sturdy frame complete with a glass cover and positive and negative terminals on the back.
How much sunlight energy does our PV cell absorb? Unfortunately, the most that our simple cell could absorb is around 25 percent, and more likely is 15 percent or less. Why so little?
Besides Single-crystal Silicon... Single-crystal silicon isn't the only material used in PV cells. Polycrystalline silicon is also used in an attempt to cut manufacturing costs, although resulting cells aren't as efficient as single crystal silicon. Amorphous silicon, which has no crystalline structure, is also used, again in an attempt to reduce production costs. Other materials used include gallium arsenide, copper indium diselenide and cadmium telluride. Since different materials have different band gaps, they seem to be "tuned" to different wavelengths, or photons of different energies. One way efficiency has been improved is to use two or more layers of different materials with different band gaps. The higher band gap material is on the surface, absorbing high-energy photons while allowing lower-energy photons to be absorbed by the lower band gap material beneath. This technique can result in much higher efficiencies. Such cells, called multi-junction cells, can have more than one electric field.
Energy Loss in a Solar Cell
Visible light is only part of the electromagnetic spectrum. Electromagnetic radiation is not monochromatic -- it is made up of a range of different wavelengths, and therefore energy levels. (See How Special Relativity Works for a good discussion of the electromagnetic spectrum.)
Light can be separated into different wavelengths, and we can see them in the form of a rainbow. Since the light that hits our cell has photons of a wide range of energies, it turns out that some of them won't have enough energy to form an electron-hole pair. They'll simply pass through the cell as if it were transparent. Still other photons have too much energy. Only a certain amount of energy, measured in electron volts (eV) and defined by our cell material (about 1.1 eV for crystalline silicon), is required to knock an electron loose. We call this the band gap energy of a material. If a photon has more energy than the required amount, then the extra energy is lost (unless a photon has twice the required energy, and can create more than one electron-hole pair, but this effect is not significant). These two effects alone account for the loss of around 70 percent of the radiation energy incident on our cell.
Why can't we choose a material with a really low band gap, so we can use more of the photons? Unfortunately, our band gap also determines the strength (voltage) of our electric field, and if it's too low, then what we make up in extra current (by absorbing more photons), we lose by having a small voltage. Remember that power is voltage times current. The optimal band gap, balancing these two effects, is around 1.4 eV for a cell made from a single material.
We have other losses as well. Our electrons have to flow from one side of the cell to the other through an external circuit. We can cover the bottom with a metal, allowing for good conduction, but if we completely cover the top, then photons can't get through the opaque conductor and we lose all of our current (in some cells, transparent conductors are used on the top surface, but not in all). If we put our contacts only at the sides of our cell, then the electrons have to travel an extremely long distance (for an electron) to reach the contacts. Remember, silicon is a semiconductor -- it's not nearly as good as a metal for transporting current. Its internal resistance (called series resistance) is fairly high, and high resistance means high losses. To minimize these losses, our cell is covered by a metallic contact grid that shortens the distance that electrons have to travel while covering only a small part of the cell surface. Even so, some photons are blocked by the grid, which can't be too small or else its own resistance will be too high.
Now that we know how a solar cell operates, let's see what it takes to power a house with the technology.
Solar-powering a House
What would you have to do to power your house with solar energy? Although it's not as simple as just slapping some modules on your roof, it's not extremely difficult to do, either.
First of all, not every roof has the correct orientation or angle of inclination to take advantage of the sun's energy. Non-tracking PV systems in the Northern Hemisphere should point toward true south (this is the orientation). They should be inclined at an angle equal to the area's latitude to absorb the maximum amount of energy year-round. A different orientation and/or inclination could be used if you want to maximize energy production for the morning or afternoon, and/or the summer or winter. Of course, the modules should never be shaded by nearby trees or buildings, no matter the time of day or the time of year. In a PV module, even if just one of its 36 cells is shaded, power production will be reduced by more than half.
If you have a house with an unshaded, south-facing roof, you need to decide what size system you need. This is complicated by the facts that your electricity production depends on the weather, which is never completely predictable, and that your electricity demand will also vary. These hurdles are fairly easy to clear. Meteorological data gives average monthly sunlight levels for different geographical areas. This takes into account rainfall and cloudy days, as well as altitude, humidity, and other more subtle factors. You should design for the worst month, so that you'll have enough electricity all year. With that data, and knowing your average household demand (your utility bill conveniently lets you know how much energy you use every month),there are simple methods you can use to determine just how many PV modules you'll need. You'll also need to decide on a system voltage, which you can control by deciding how many modules to wire in series.
You may have already guessed a couple of problems that we'll have to solve. First, what do we do when the sun isn't shining?
Solving Solar-power Issues
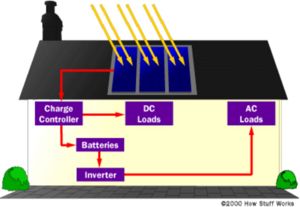
Certainly, no one would accept only having electricity during the day, and then only on clear days, if they have a choice. We need energy storage -- batteries. Unfortunately, batteries add a lot of cost and maintenance to the PV system. Currently, however, it's a necessity if you want to be completely independent. One way around the problem is to connect your house to the utility grid, buying power when you need it and selling to them when you produce more than you need. This way, the utility acts as a practically infinite storage system. The utility has to agree, of course, and in most cases will buy power from you at a much lower price than their own selling price. You will also need special equipment to make sure that the power you sell to your utility is synchronous with theirs -- that it shares the same sinusoidal waveform and frequency. Safety is an issue as well. The utility has to make sure that if there's a power outage in your neighborhood, your PV system won't try to feed electricity into lines that a lineman may think is dead. This is called islanding.
If you decide to use batteries, keep in mind that they will have to be maintained, and then replaced after a certain number of years. The PV modules should last 20 years or more, but batteries just don't have that kind of useful life. Batteries in PV systems can also be very dangerous because of the energy they store and the acidic electrolytes they contain, so you'll need a well-ventilated, non-metallic enclosure for them.
Although several different kinds of batteries are commonly used, the one characteristic they should all have in common is that they are deep-cycle batteries. Unlike your car battery, which is a shallow-cycle battery, deep-cycle batteries can discharge more of their stored energy while still maintaining long life. Car batteries discharge a large current for a very short time -- to start your car -- and are then immediately recharged as you drive. PV batteries generally have to discharge a smaller current for a longer period (such as all night), while being charged during the day.
The most commonly used deep-cycle batteries are lead-acid batteries (both sealed and vented) and nickel-cadmium batteries. Nickel-cadmium batteries are more expensive, but last longer and can be discharged more completely without harm. Even deep-cycle lead-acid batteries can't be discharged 100 percent without seriously shortening battery life, and generally, PV systems are designed to discharge lead-acid batteries no more than 40 percent or 50 percent.
Also, the use of batteries requires the installation of another component called a charge controller. Batteries last a lot longer if care is taken so that they aren't overcharged or drained too much. That's what a charge controller does. Once the batteries are fully charged, the charge controller doesn't let current from the PV modules continue to flow into them. Similarly, once the batteries have been drained to a certain predetermined level, controlled by measuring battery voltage, many charge controllers will not allow more current to be drained from the batteries until they have been recharged. The use of a charge controller is essential for long battery life.
The other problem besides energy storage is that the electricity generated by your PV modules, and extracted from your batteries if you choose to use them, is not in the form that's used by the electrical appliances in your house. The electricity generated by a solar system is direct current, while the electricity supplied by your utility (and the kind that every appliance in your house uses) is alternating current. You will need an inverter, a device that converts DC to AC. Most large inverters will also allow you to automatically control how your system works. Some PV modules, called AC modules, actually have an inverter already built into each module, eliminating the need for a large, central inverter, and simplifying wiring issues.
Throw in the mounting hardware, wiring, junction boxes, grounding equipment, overcurrent protection, DC and AC disconnects and other accessories and you have yourself a system. Electrical codes must be followed (there's a section in the National Electrical Code just for PV), and it's highly recommended that the installation be done by a licensed electrician who has experience with PV systems. Once installed, a PV system requires very little maintenance (especially if no batteries are used), and will provide electricity cleanly and quietly for 20 years or more.