FreeEnergyLesson4
LESSON 4: TEMPERATURE DEPENDENCE OF GIBBS ENERGY
The temperature dependence of Gibbs energy, (G/T)p = -S, was given by eq (3.5). For a chaemical reaction with all species in the standard state we can write:
d∆IGo/dT = -∆ISo (4.1)
since ∆IGo implies that all reactants and products are at standard pressure.
From the two expressions for ∆IGo:
∆IGo = ∆IHo - T∆ISo from eq.(2.1)
and:
∆IGo = -RT ln K from eq.(3.17)
we shall derive a relationship between ∆IHo and In K. Differentiating eq. (3.17) with respect to
T and changing signs we obtain:
∆So = R In K + RT dIn K/dT
Multiplying with T, and replacing RT In K with ∆IGo, give:
T∆ISo = -∆IGo + RT2 dIn K/dT
Using eq. (2.1) and rearranging, we obtain:
d ln K/dT = ∆THo/RT2 (4.2)
This equation is often called the van t'Hoff equation.
Since d ln K = - dT/T2, eq. (4.2) can be given the form:
d ln K/d(l/T) = -∆THo/R(4.3)
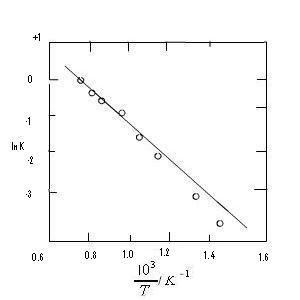
When plotting In K as a function of VT we obtain -∆rHo/R as the slope of the curve, see Fig. 4.1. The variation in ∆rHo with temperature is given by eq (5.9)in 1st law of thermodynamics lesson 5
∆rHoT = ∆rHo298 +∆rCPodT
The integral in the eq. ∆rHoT = ∆rHo298 + ![]() ∆rCpodT will usually have a small value compared to ∆rHo298 except when the temperature range is very large. This means that the curve in Fig. 4.1 is approximately a straight line. ∆rHo for a reaction is found from a plot similar to the one in Fig. 4.1. The experiment shows that the variation in ∆rHo with temperature can be ignored.
∆rCpodT will usually have a small value compared to ∆rHo298 except when the temperature range is very large. This means that the curve in Fig. 4.1 is approximately a straight line. ∆rHo for a reaction is found from a plot similar to the one in Fig. 4.1. The experiment shows that the variation in ∆rHo with temperature can be ignored.
Fig. 4.1. The temperature dependence of the equilibrium constant for the reaction: CO2(g) + H2 (g) = CO (g) + H2O (g).
The variation with temperature of ∆rSo for a reaction is also usually very small, and therefore ∆rGo for a reaction is usually a practically linear function of temperature. For many purposes one will obtain results with sufficient accuracy by replacing the equation:
∆rGoT = ∆rHoT - T∆rSOT
with the approximate equation:
∆rGoT = ∆rHo298 - T∆rSo298 (4.4)
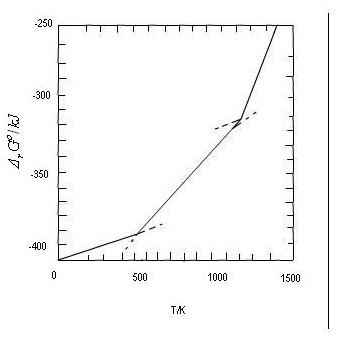
Equation (6.45) represents a straight line with the slope ∆rSo298. If the line is extrapolated to the absolute zero, the intercept with the ordinate gives ∆rHo298
At temperatures of transition, there is a change in ∆rHo and a corresponding change in ∆rSo.
Equation (4.8)in Entropy lesson 4, gives the relation between ∆fusSo and ∆fusHo at the melting point. When a transition takes place reversibly, i.e., the system and the surroundings are at the same temperature, the transition temperature, there is no change in ∆rGo. There is a break in the curve for ∆sGo as a function of T, however, since the values of ∆rHo and ∆rSo have changed by the transition. An increased slope corresponds to a phase transition in a reactant, whereas a decreased slope corresponds to a phase transition in a product. The change in slope corresponds to the entropy of transition. It is small for melting and large for boiling.
Example1
We shall calculate ∆rGo for NaCl as a function of temperature in the range 298 - 1400 K using the approximate eq. (4.4)
In a temperature range where there is no phase change we assume constant values for ∆fHo and ∆fSo. A phase change leads to changes in ∆fHo and ∆fSo.
Temperature range 298 - 371 K: Na (s) + 112 Cl2 (g) = NaCI (s)
∆fHo = - 411 kI, ∆fSo = 72 - 51 - 112x223 = - 90.5 I K-1 (see eq. 2.2)
∆fGo = (- 411 + T 90.5/1000) kJ
Temperature 371 K: Na (s) = Na (1); ∆fusH/Tfus = 3000/371 = 8 I K-l = ∆fusS (see eq. (4.8)in Entropy lesson 4)
∆fusG = ∆fusH - Tfus∆fusS = 0
Temperature range 371 - 1074 K: Na (I) + 1/2 Cl2 (g) = NaCI (s)
∆fHo = - 411 - 3 = - 414 kJ; ∆fSo = - 90.5 - 8 = - 98.5 J K-1
∆fGo = (-414 + T 98.5/1000) kJ
Temperature 1074 K: NaCl (s) = NaCl (l); fusH/Tfus = 28000/1074 = 26.1 J K-1 = ∆fS
∆fusG = 0
Temperature range 1074 - 1165 K: Na (I) + 1/2 Cl2 (g) = NaCl (I)
∆fHo = - 414 + 28 = - 386 kJ; ∆fSo =- 98.5 + 26.1 = - 72.4 J K-1
∆fGo = (-386 + T 72.4/1000) kJ
Temperature 1165 K: Na (l) = Na (g); ∆vapH/lTb = 106000/1165 = 91 J K-1 = ∆vapS = 0
∆vapG =0
Temperature range 1165 - 1400 K: Na (g) + 1/2 Cl2 (g) = NaCI (I)
∆fHo - 386 - 106 - = - 492 kJ; ∆fSo = - 72.4 - 91 = - 163.4 J K-1
∆fGo = (-492 + T 163.4/1000) kJ