Chemistry/Mendeleev
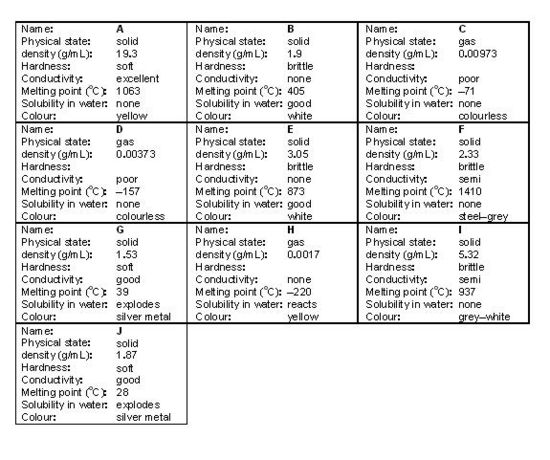
In this activity you will be given a chart containing the properties of eighteen “known” elements or compounds and a set of ten “unknown” elements or elements.
|
PROCEDURE 1. Cut out the 10 “unknown” cards below. You should notice that there are 12 possible locations to place the 10 cards — when you are finished, there will be two numbered locations which are not covered by an “unknown” card (this helps to keep you on your toes).
|
QUESTIONS
1. How were elements in Medeleev’s periodic table arranged?
2. What is Medeleev’s periodic law?
3. Why was Mendeleev able to predict the properties of elements which had not yet been discovered?
4. Do all the properties follow a smooth trend when comparing the elements or compounds in a family? Support your answer using data from your finished chart.
5. You have been called in as a highly paid consultant to the Atomic Energy Commission to predict the properties of Element 114. Although this element has not been discovered, nuclear physicists are sure that they can make it some day soon. Your task is to predict as many as you can of the properties of Element 114, which will lie directly underneath the element lead (Pb) on the periodic table. They are paying you for every property you predict, so if not sure, make a good guess.