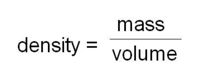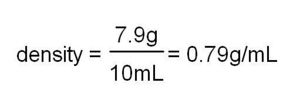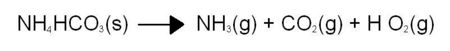Chemistry/Doing Science in the Elementary School
A modest suggestion: young kids don't gain much by being able to recite long lists of facts. Memorizing the names of the ten planets is about as relevant as memorizing the middle names of all the mothers of the past 15 Canadian Prime Ministers. Simply making pretty models of the planets is a nice art project, but is it science? No.
Science IS: an ACTIVE METHOD FOR INVESTIGATING AND EXPLAINING THE WORKINGS OF NATURE.
- a systematic process of enacting wonderment.
- a scheme for thinking about WHY things happen and testing these ideas.
- a vibrant and SELF-CORRECTING process which appeals to no authority other than the supremacy of events/facts. When new events/facts fly in the face of old and revered explanations, the explanation is discarded or modified, not the facts.
- a fluid, ever changing body of knowledge.
- a great way to use some of the arithmetic, art, questioning techniques and imagination that kids learn.
Science for kids:
Contents
[hide]- 1 SHOULD
- 2 SHOULD NOT
- 3 The role of a teacher OF science is to guide students through a process which involves using past knowledge in:
- 4 Incidentally:
- 5 Why Do Chemistry (and Physics) in the Elementary School?
- 6 What to look for when choosing an "investigation".
- 7 Some "Chemistry" Things to Try and Why They Happen(A Teachers Guide)
- 7.1 The Mysterious U-Tube
- 7.2 The Reaction Between Aluminum and Cupric Chloride
- 7.3 Heating "Smelling Salts" (teacher demonstration)
- 7.4 Burning a Sugar Cube Coated with Cigarette Ashes
- 7.5 Heavy Water
- 7.6 The Dissolving of Washing Soda and Baking Soda in Water
- 7.7 A Cool Liquid
- 7.8 75 + 25 = 95 : Chemical Math
- 7.9 The Mysterious Sinking Ice Cube
- 7.10 Paper Chromatography
- 7.11 Smoke Rings in Water
- 7.12 Flame Tests
- 7.13 A Whole Mess of Simple Physics Experiments Guaranteed to Interest Kids
- 8 SAFETY CONCERNS REGARDING THE CHEMICALS USED
SHOULD
- catch their attention (novel or new)
- be colourful
- be visible
- scream for explanations (arguments/evidence/logic)
- be hands on (active)
- teach processes
- leave them wanting to know more
- let them connect the results of one investigation to another
- stress various ways to reconstruct meaning (tables, drawings, graphs, etc.)
SHOULD NOT
- bore them out of their minds
- be "gray"
- be theoretical
- be presented as a "canned" explanation
- be "stand quiet and watch" (passive)
- teach collections of facts
- leave them glad "that Unit is over with"
- be left as isolated conclusions
- always be presented in the same manner
The role of a teacher OF science is to guide students through a process which involves using past knowledge in:
an awakening of wonder to recognize that something special is going on,
- the collecting of relevant observations and ways to display observations,
- the posing of possible explanations: hypothesizing or generalizing,
- the construction of ways to test the explanations and the concept of what constitutes an explanation,
- the interpretation of the results of testing,
- the drawing of conclusions with respect to the "fitness" of our explanations,
- understanding that an explanation is a "best fit" concept, rather than an absolute, and
- the net result - the "so what" of it all!
Incidentally:
A HYPOTHESIS is a tentative suggestion which attempts to EXPLAIN why something happened,
A THEORY is a set of hypotheses which have survived the ordeal of extensive testing and which has gained a certain "reputation" for its ability to explain a wide variety of events similar to what caused the original hypotheses to be put forward. Theories can NEVER be proven, simply tested with all possible situations which people can envision. On a regular basis in science, a new fact emerges which causes cherished theories to lie in tatters, while scientists mutter "well, back to the drawing board."
A LAW is simply a statement of what is always observed to happen in a given situation. (The law of gravity says, in effect, that objects will move toward nearby objects which have mass, or more simply: if you drop a rock, it tries to fall to the centre of the earth.) Theories NEVER become LAWS because theories are EXPLANATIONS and laws are statements of what is always seen to occur (with no explanation as to why). There are several theories as to why the law of gravity works.
Why Do Chemistry (and Physics) in the Elementary School?
Bean seeds are fun to play with, but give verrrrrry slow results. Bean seeds sometimes seem to die just for the fun of it, and grow when they have no right to do so, but otherwise they normally insist on doing almost exactly what any moderately observant kid knows they will do. Gardens are not a miracle available only to a school classroom. Biology tends to be overdone because the available materials are often dirt cheap and readily available (remember that this is a CHEMIST doing the writing).
Chemistry and physics investigations provide kids with the opportunity to do things in an immediate manner. They get quick "feed back"; they get presented with "mini-mysteries"; they get excited by the highly visual and sometimes dramatic aspects ("Wow, look at that bean seed go!" is not a comment you will hear too often); they get a chance to improve their abilities to observe, reason, compare, predict, conclude and present results.
What to look for when choosing an "investigation".
(Not all of these may exist in a particular investigation.)
Does the process do something completely unexpected?
- Does the process promote a sense of wonder?
- Does the process exhibit changes in colour, temperature, phase, etc.?
- Does the process allow students to make interesting guesses, based on their observations and personal experiences, about what is happening and why it is happening?
- Does the process have a definite "threshold", such that it is clearly evident when something is happening and when it is not?
- Does the process have a simple enough explanation to be understood by a kid who is suitably prepared?
- Does the investigation lend itself to "extensions" which allow a kid to go beyond what was seen to create general ideas and to ask "what if"? Better still, can the kid carry out one or more of these extensions?
Some "Chemistry" Things to Try and Why They Happen(A Teachers Guide)
NOTE: A "*" indicates some safety precautions are in order; see the safety section at the end. NO highly hazardous chemicals are used here. (Incidentally, common household salt has an 8 page safety handout from the WHMIS boys, so don't get scared off by the terror phrase "SAFETY CONCERNS REGARDING THE CHEMICALS USED".)
The Mysterious U-Tube
A 1 meter piece of clear vinyl tubing (5/16" inside diameter; available from Canadian tire for $5/10 foot piece) is mounted or held in a U-shape. The tube arrangement is approximately half filled with water. Then, carefully pour methyl hydrate (*) (easily obtained from a hardware store) into ONE side of the tube arrangement. Keep pouring until the methyl hydrate is almost up to the top of one tube. The liquid level is higher on the methyl hydrate side.
Great for hypothesizing! Blowing very gently on the higher tube shows that the tube is not "blocked" at the bottom. Kids can make a prediction which they can test by using water and the bottle of methyl hydrate (which you just happen to have on hand.)
Explanation: Methyl hydrate is lighter (lower "density") than water. Imagine a teeter-totter with one sack of sand on one end and a pile of feather pillows on the other end. It takes a higher pile of pillows (and methyl hydrate) to balance the downward push at the other end from the sandbag (and water).
Extensions:
a) How can a person measure the densities? (suggestion: which volume of 2 different liquids has the greatest mass (weight") Technically:
For example: 10 mL of methyl hydrate is found to have a mass of 7.9 g. Hence:
b) Can this method be used with, say, water and vinegar? Salt water (poured in first) and tap water? Hey! Why is salt water heavier? (Extra stuff dissolved in the water makes it heavier) Water and vegetable oil? (careful here, because the oil is difficult to clean out - a little detergent or methyl hydrate will help if vegetable oil is used)
The Reaction Between Aluminum and Cupric Chloride
Into a 100 mL beaker (or baby-food jar) is placed about 50 mL of cold tap water. A small scoop (about 1 teaspoon) of cupric chloride (*) is dumped into the water. Observe. Lightly crumple up a 10 cm square of aluminum foil and place it in the solution. Push the foil down into the solution with a plastic coffee stirrer. Observe! (Kids will see the foil blacken where the foil has been creased, then bubbles will appear on the surface of the foil, then the black spots enlarge and start to look like "rust" while the beaker starts to get warmer. After a minute or so, the foil has disintegrated into small pieces and then more or less disappears). Feel the outside of the container. When the reaction is finished, pour the liquid down the sink (the products are all harmless), but keep the red solid. Fish out as much of the red stuff as possible and dry it between layers of paper towel. Then take a hammer, say, and slowly "grind" the red stuff against a metal surface. Look at the red stuff after grinding.
Note: since pure cupric chloride is quite expensive, a much cheaper alternative is to mix equal amounts of table salt and powdered copper sulphate.
Explanation: The "cupric chloride" dissolves to give pretty blue copper ions. The aluminum is attacked by the copper ions (aided by the chloride ions), causing the aluminum to go into solution as aluminum ion and the copper ions to come out of solution as pure copper metal (the red stuff). "Rust" is a term which is reserved for the product of iron reacting with water and air, but if the kids say it is rust, who cares! When the copper ions react and come out of solution, the blue colour disappears. The aluminum ions form a milky gray solution of aluminum hydroxide (which is related to underarm deodorant). When the powdered copper is polished by rubbing between pieces of metal, the shiny copper colour (like a new penny) appears. The gas that comes off is NOT chlorine, so relax; in fact it is just a little hydrogen gas which comes off so slowly that there is no possible hazard. The reaction also produces heat (is "EXOTHERMIC"; that is heat EXits from the reaction). All the processes come to an and at the same time - heat production, bubbling, production of red stuff and loss of blue colour - signifying the end of the reaction.
Extension: Try trapping the gas and testing it with a glowing split (produces a "popping" sound). Will other metals beside aluminum cause a reaction to occur? Try an iron nail (copper coating on the nail), a penny (no effect - copper does not react with copper ions), a piece of galvanized metal (get a copper coating on the metal) [galvanized means zinc metal is plated onto the underlying metal], a nickel (may or may not react), a Looney (probably no effect), a piece of silver (no effect). What do you get if you allow the liquid from the reaction to evaporate to dryness? (a gray-white residue of aluminum hydroxide - this is where the missing aluminum went to)
Heating "Smelling Salts" (teacher demonstration)
A little ammonium bicarbonate (*), say 1/8 of a teaspoon, is placed in the bottom of a large test tube and a cork is lightly placed into the end ot the tube (note - a gas will be produced, so make sure the cork is just resting in the end, not tightly jammed in). [CAUTION: This stuff is sometimes called "smelling salts", and has a VERY strong odour of ammonia - which is why it quickly revived great grandma when it was held under her nose as a result of her having fainted when she saw some brazen hussy expose an ankle while stepping off a curb.] Hold the upper end of the test tube with either a test tube holder or a "rope" of damp paper towel. Heat the lower end of the test tube with an alcohol lamp (say). You will see the white solid start to jump around and the amount of solid will steadily decrease until it completely disappears over about a one minute period. Simultaneously, a clear, colourless liquid starts to condense on the inside of the tube. [CAUTION: the liquid reeks of ammonia, so if smelling it, do so carefully!] The contents of the test tube may be washed down the sink.
Explanation:Ammonium bicarbonate decomposes easily if simply left in the air. Heating simply increases the rate of this decomposition reaction. The reaction is:
or, in words: smelling salts produces ammonia, carbon dioxide and water. The water comes off as a vapour and escapes during the normally-slow decomposition in the open air, but in the demonstration above the water is produced in a closed container and condenses into liquid water (we produce a high concentration of water inside the tube). The ammonia gas produced is highly soluble in the water produced, forming a solution of "household ammonia".
Extensions:If litmus paper is available, test the liquid produced inside the tube to see if it is acidic, neutral or basic. (Litmus is reD in aciD and Blue in Base.). What happens if a tiny quantity of salt is heated? (nothing) What if a tiny quantity of mothballs is heated? (care - flammable) (The mothballs, melt then boil, and recondense at the top of the tube as a solid which is essentially unchanged.)
Burning a Sugar Cube Coated with Cigarette Ashes
Hold a sugar cube in the flame of a candle or an alcohol burner. (Use a pair of tweezers, pliers, etc.) Nothing happens apart from a little melting. Rub cigarette ashes over the surface of a sugar cube and place the cube in a flame again. The cube burns fairly rapidly, with a sputtering appearance.
Explanation: Tobacco extracts and concentrates uranium from the soil in which the plant grows. Uranium undergoes radioactive decay to produce a very small amount of radioactive thorium (thorium is another chemical element; symbol Th). This thorium is a CATALYST which accelerates the rates of burning of the sugar. I doubt that anyone really knows WHY thorium acts as a catalyst here, it just does. Incidentally, radioactive thorium is constantly decomposing to the element "radon", which is a radioactive gas that is sucked into the lungs when a cigarette is smoked. Radon is strongly suspected as a cause of lung cancer on its own, over and above the cancer-causing effects of the rest of the junk in cigarette smoke. A great demonstration for helping to convince kids not to smoke! (It worked beautifully on a 16 year-old girl who need a little extra convincing to stop smoking.)
Necessary Extensions: What happens if other types of ashes are tried, such as paper ash or wood ash? (nothing, but kids should try this to see that cigarette ashes are unique)
Heavy Water
Allow a sugar cube (or two) to sit in about 50 mL of water in a small glass container (a 100 mL beaker is ideal) for half an hour or so. DO NOT STIR. After the sugar has more or less dissolved, slowly tilt the container back and forth while looking through the container at a light source. An effect similar to "heat-waves" will be seen moving across the bottom.
Explanation: When sugar (or any other soluble substance) dissolves without being stirred, the water at the bottom of the container is "heavier" (has a higher density) than the water above it due to the added sugar. Light passing through a liquid is bent (refracted); the greater the density of the liquid, the greater the bending. Hence, when the sugar solution is agitated by tilting, various density layers are produced and the light is bent by varying amounts depending on the particular density of the solution at that point (which in turn depends on how much the low density water layer above is mixed with the higher density sugar solution below). The result is a "ripple" effect that looks like heat waves coming up from a road. Incidentally, when the air above a road is heated, the air expands and has a lower density (less air "molecules" in a given volume). Since warm air rises (lower density things try to float on higher density things), we get a mixing of air layers having different densities and the light is bent by different amounts as it passes through the air, producing "heat waves".
The Dissolving of Washing Soda and Baking Soda in Water
If a spoonful of washing soda (*) is added to about 50 mL of room temperature water and stirred, the container feels noticeably warmer. If a spoonful of baking soda is stirred into about 50 mL of room temperature, the container gets somewhat cooler.
Explanation: In order to break a "bond" holding two atoms together, energy must be added from the surroundings, in the same way that you have to add energy to rip apart two pieces of Velcro. The result of taking heat from the surroundings is to make the surroundings feel COOLER. Conversely, when two atoms join together, they give off energy to their surroundings and the surroundings feel WARMER. When a solid substance dissolves, two opposing process take effect at the same time:
a) Heat is absorbed from the surroundings and used to break one molecule away from another. (this produces a cooling tendency)
b) Heat is given off to the surroundings when water "bonds" to the molecules. (this produces a warming effect)
Hence, if a container feels warmer when a chemical is dissolved in water, we know that more heat is given off than is absorbed. Conversely, if a container feels cooler when a chemical is dissolved in water, we know that more heat is absorbed than is given off. If no temperature change is felt, then the cooling and warming effects must balance out each other.
Extensions: Is there any temperature change when salt is dissolved? (not noticeable) Sugar? (not noticeable) What about copper sulphate? (what was observed in #2, above?)
A Cool Liquid
Have students open their hand, palm upwards. Place a few drops of methyl hydrate (*) on the palm and instruct them to blow gently on the liquid. Their hands will feel cold. (The methyl hydrate is absolutely harmless, but might leave the skin with a slightly white look because the methyl hydrate dissolves skin oil.)
Explanation: Evaporation occurs when molecules gather up enough energy to escape from their fellow molecules in the surrounding liquid. (This is an ENDOTHERMIC process in which heat ENters the liquid from the hand. The energy entering the liquid is used to allow one molecule to break free of its neighbours.) Only the "hottest" molecules (having the most energy) will be able to escape, so that the remaining molecules have less energy, on the average, than they used to. Since the overall energy of the liquid has been lowered, the liquid feels cool since it has a greater tendency to "suck heat" from the hand.
Extensions: Try this with water. This is the basis of holding a wet finger up in the air to see which way the wind is coming (it feels colder on the side where the wind is coming from). Try this with vegetable oil. (Little or no effect since the oil doesn't evaporate and hence doesn't cause a cooling effect. In fact, the oil-coated finger feels WARMER since the oil acts as an insulator, prevents the body's heat from escaping and causes a buildup of heat on the finger.)
75 + 25 = 95 : Chemical Math
Take a vinyl tube (1 m), firmly stoppered at one end and 3/4 fill it with water. Then slowly add methyl hydrate (*), in such a way that very little mixing occurs where the liquids meet. Fill to slightly overflowing and carefully insert a stopper at the top. There should be no air bubbles observable at the top end (a very small one won't hurt). Mix the liquids by tipping the tube upside down repeatedly, waiting each time until a "bubble" which appears out of nowhere moves to the top of the tube. After several mixings (8-10) the tube will now be about 95% full!
Explanation: If a big box with a lid is filled with basketballs, there is still room to add many tennis balls. In the same way, the tiny water molecules are able to "fit between" the larger methyl hydrate molecules, giving rise to a smaller total volume than would be expected by simply adding together the volumes occupied by the water and methyl hydrate molecules. (Suggestion: use the largest diameter tubing you can get for easier mixing.)
Extensions: What if methyl hydrate is mixed with salt water? (I don't know either - try it and let me know) What about methyl hydrate and vinegar? (I doubt you will see any effect) What about water and vegetable oil? (no effect is expected) [this will be harder to clean out - use detergent or a little methyl hydrate] The vegetable oil demonstrates a new effect: liquids that DO NOT mix together - "oil and water don't mix".
The Mysterious Sinking Ice Cube
Place an ice cube in a glass of water. Observe. Put a second ice cube in a second glass containing a clear liquid (methyl hydrate *). Observe.
Explanation: Ice has a density of 0.92; water has a density of 1.00. Lighter things float on heavier things, so the ice floats in water. Methyl hydrate has a density of 0.79, so the ice sinks. If a kid thinks the ice cubes are different, fish the cube out of the methyl hydrate and put it into the water. This could go nicely with a little exercise in which kids weigh 10 mL of water, 10 mL of methyl hydrate, and a chunk of wood which measures 1 cm x 1 cm x 10 cm (and therefore has a volume of 10 cm3; that is, 10 mL). They can then calculate the density (see #1, above). Wood floats in water, leading to a discussion of how this is related to density.
Extensions: How high does a piece of wood float in water? How high does it float in methyl hydrate? (For the mathematically advanced kid, they might be able to see that there is a relationship between the difference of the densities of the liquid and the solid, and how high out of the liquid the solid floats.)
Paper Chromatography
Take a piece of thick, absorbent paper (filter paper works very well) and cut it into a strips about 1 cm x 15 cm. (The size is not really too important.) About 1 cm from one end of a paper strip, make a circular blob of ink with a felt pen (or Quink writing ink). Suspend the paper so that it hangs down into the water (ink end down) and is immersed about 1/2 cm into the water. The water will climb up the paper and makes the ink move up as well. As the ink moves up the paper, the ink will separate into various different colours. Pull the paper out before the water gets to the top of the paper and let it dry.
Explanation: Most inks are made up of two or more different dyes. Each dye has a tendency to stick to the paper and a tendency to dissolve in the water passing over the paper. Each dye acts differently so that those sticking more to the paper remain closest to the starting point while those having the greatest tendency to dissolve in the water will move higher on the paper. This process is called "PAPER CHROMATOGRAPHY" and is used extensively to analyze mixtures.
Smoke Rings in Water
Dissolve a teaspoon of washing soda (*) in about 50 mL of water (the amounts are not very important). Dissolve a spoonful of cupric sulphate (*) in about 50 mL of water. Use a dropper to add cupric sulphate solution to the washing soda solution in a dropwise fashion. Each time a drop of cupric sulphate is added, a little "ring" appears in the solution. Intrigues kids (and adults) for hours. A beautiful sight!
Explanation: Cupric sulphate dissolves to form copper ions:
Washing soda dissolves to form carbonate ions:
(The foregoing was for the benefit of those who remember taking a chemistry course.) Now down to the nitty-gritty. When the two soluble ions (copper and carbonate) meet each other in solution, they are immediately attracted and join together to form a solid, which appears as a little puff of flour-like material in the solution. The process of forming a solid, starting with two dissolved substances is called PRECIPITATION. The fact that a solid is formed can be seen if the mixture is left to settle; the solid goes to the bottom because it is more dense than the liquid.
Extensions: Do you get a reaction which forms solids ("smoke rings") with other mixtures of substances? Try salt (no effect normally), baking soda (probably gives a precipitate of pale blue copper carbonate with copper sulphate), smelling salts (may bubble in copper sulphate and/or may give a precipitate of pale blue copper carbonate), vinegar (no precipitate with ammonium bicarbonate, but should form bubbles of carbon dioxide), the water in which a nail was left to rust for a week or so (may form a precipitate of brownish iron carbonate when washing soda is added). (Hint: a little cupric sulphate mixed with household ammonia may be interesting - you might see a clear purple layer above a fluffy whitish-blue cloud). If household ammonia is mixed with an equal volume of vinegar, most (if not all) of the ammonia and vinegar smell will disappear because the ammonia and vinegar react with each other to form ammonium acetate, which is odourless.
Flame Tests
Make a very small loop (about 3-4 mm across) in a piece of wire. Dip the loops in some cupric sulphate solution and put the loop in the flame of an alcohol lamp. Make another loop and try washing soda solution. Make yet another loop and try a solution of borax.
Explanation: Some chemicals emit a distinct colour when burned in a flame. When a "flame test" is performed as described above, it becomes possible to identify the presence of a particular substance. This is part of the process called chemical analysis. Copper typically gives an emerald flame, sodium gives an orange-yellow flame, borax gives a yellowish-green flame. This is the same effect we see in "Yule logs" which burn to give different colours, or fireworks which give distinctive colours when they explode.
Extensions: Try to see if the results are different with different kinds of wire (for example: copper, steel, aluminum). [Copper wire will probably give a green tint to the flame, the others will have no effect.] Contact your local high school for samples of lithium chloride (unbelievably gorgeous!), calcium chloride, potassium chloride and strontium chloride. Calcium acetate works well and can be prepared by dissolving some white chalk dust in vinegar. How dilute a solution can be used and still allow you to see the colour from the copper?
A Whole Mess of Simple Physics Experiments Guaranteed to Interest Kids
Contact Gordon Gore (962 Sicamore Drive, Kamloops, B.C. V2B 6S2, (604)-579-5722. Gordon has instructed elementary student teachers at University College of the Cariboo in how to teach science. He has prepared a sourcebook called "Science experiments for Elementary Students", subtitled "A Sourcebook of 'Hands on', 'Minds on' Experiments for Young People to Do".
This is incredible stuff from a teacher who won a Master Teacher award, for good reason. Thirty-seven experiments are described, and include a special section for the student, a "Top Secret! For the Teacher Only" section which describes the background (so kids don't leave you at a loss for explanations), Extensions and questions to think about which cause kids to want to do more on their own, and a list of equipment needed. This should be a part of every teacher's resources - one per teacher would be best. Includes many cartoons that kids appreciate. Gordon is a master of the quick pun, which kids really like.
SAFETY CONCERNS REGARDING THE CHEMICALS USED
The chemicals used are no more hazardous (AND NO LESS SO) than many chemicals that kids may be exposed to around the normal household. A kid that drinks window cleaner, bleach, washing liquids or detergents is in serious danger. None of the chemicals mentioned below are particularly hazardous to the touch, but washing of hands after touching any chemical is always necessary, even around the house.
- Methyl Hydrate (also called wood alcohol, methanol, or methyl alcohol). Do NOT use any of the terms involving the word "alcohol", in case some kid tries to drink the stuff. It is poisonous if consumed and causes a hot sensation in the mouth. 50 mL of this stuff will cause blindness. It is also flammable, which is why it is used as "fondue fuel". If someone DOES drink the stuff, get them to throw up immediately, and get them to a hospital.
- Copper Sulphate (also called "bluestone", cupric sulphate or copper (II) sulphate). This stuff is toxic if swallowed, but has very disagreeable metallic bitter taste which would increase the likelihood of a kid immediately spitting it out or "throwing up". Continued handling can cause skin irritation. If it gets into a cut, it will sting.
- Ammonium Bicarbonate (also called ammonium hydrogen carbonate or "smelling salts"). The greatest hazard is the intense ammonia smell always present, which requires very careful smelling so that you don't get a "real nose-full". Keep firmly sealed when not being used to prevent it from decomposing and escaping. (If tightly sealed, the presence of the gases produced by slight decomposition completely inhibits the further decomposition of the remaining solid.)
- Washing Soda (also known as sodium carbonate). This stuff is somewhat caustic and is toxic if ingested in sufficient quantities (although it has a very bitter and disagreeable taste). Not very hazardous to touch, but wash after touching since it might cause a skin rash.