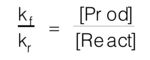Chemistry/Chem Teaching Strategies
CHEMISTRY TEACHING STRATEGIES
Contents
[hide]A. Teaching Tips and Strategies
Stress HOW to study. Many students cannot write down the steps needed to perform a particular procedure and don’t understand the importance of being able to work a problem step by step. Hand out the document “Study Notes” and discuss the importance of making good study notes.
Chemistry 11
- Start immediately in Chem 11 to show how to analyze a problem and look for key words or concepts that identify a type of problem. Students who lack such skills NEVER do well in the physical sciences. Approach all new problems in such an analytical manner. Students learn what you model. Stress an initial careful reading to identify the unknown, relevant equations, relevant relationships and assumptions.
- When in doubt, assign MORE homework, not less. A key indicator of successful schools is that teachers in these schools DEMAND a great deal of work from their students on a daily basis.
- Students have been subjected to safety warnings for several years through Science 8, 9 and 10, even though they frequently are given no chemicals more hazardous than baking soda, salt and vinegar. I usually started my traditional safety lecture by admitting that most of the chemicals they were using could have been drunk with little ill effect. Then I would drip a little concentrated sulphuric acid down a paper towel and watch their wide-eyed amazement as the paper turned black and disintegrated. I would then point out that this was only paper and the effect on skin was worse – any questions?!! Make it clear that Chem 11 will require the highest level of safety to avoid personal injury. Make it clear that every year in North America several students (and sometimes their teachers) are seriously hurt or killed in high school Chemistry labs and that your job and theirs is to get through the year safely.
- When teaching what “something is”, also teach “what it is not”. This practice will help to eliminate confusing misunderstandings as kids struggle to make sense of a new concept. For example, density is just the amount of mass crammed into a given volume. It is not how heavy something is. Another example:
- Have students mimic water molecules with flapping arms (Head = oxygen, fists = hydrogens). They demonstrate vibration, rotation and translational motion individually. Teach combines all three motions and runs across front of room, flapping arms and spinning around. Students won’t forget this one!
- Unit conversion flash cards: Make up a series of cards which represent the unit conversions needed to carry out various operations. The top half of each card is the upper part of the fraction, the bottom half (below a heavy black line) is bottom part of fraction. The ratio is reversed on back side (arranged so that when student flips the card “top to bottom” the back side is properly seen. Students place cards beside each other, as is or flipped, to carry out multi–step conversion. Very visual and works great for some students.
- Addition of uncertain numbers: a man said a dinosaur bone was 55 000 003 years old because 3 years previously a geologist said the bone was 55 000 000 years old.
- Tin metal comes in an alpha and beta form. Apocryphal (?) story exists that Napoleon’s army marched into Russia wearing shiny tin buttons. The severe winter caused a phase change from hard alpha tin to gray powdery beta tin. Coats, pants and suspenders were left without buttons, contributing to the defeat of the army – chemistry saves the world again!
- Have students “guess”timate the length of time needed to count out Avogadro’s Number of atoms, 10 atoms/ second, using a class of thirty. Make all stand and ask them to sit if they think the count is completed in less than 1 hr, 1 day, 1 week, etc. Most will sit long before 10 years (peer pressure!) [It takes 6.4 x 1013 years]
- When naming inorganic compounds, students do poorly when they do not see clearly when to switch from one naming method to another. Be sure they have a clear set of rules since naming is partly “art”.
- Show students how to balance equations using fractions or start with 12 of each molecule (if suspected to be difficult.
- Have students grow crystals at lunch hour. This is a wonderful way to get to know students and let them experience the wonder of crystals. (You also get to teach them vast amounts of crystallography because they really want to understand what is happening as their crystals grow. Chemistry at its best!).
- Use “2–minute quizzes” to check homework. Give quick feedback on progress; marked by randomly assigning to another student (who signs bottom); randomly checked for marking accuracy – mismarked paper means “marker” gets zero.
- The Romans used lead water pipes (analyses of their bones shows massive lead concentrations, aided by their use of lead(II) acetate – “sugar of lead” – to sweeten their wine.) From their word for lead – “plumbum” – comes the word “plumbing”.
- Students typically are confused by the differences between a hypothesis, a theory and a law. Some mistakenly believe that a hypothesis becomes a theory after being tested and eventually becomes a law when the theory is proven to be true. Obviously this is ridiculous but check their perceptions because many seem to hold this belief.
- Malleability: a lead balloon has actually been made. Lead is very malleable and can be flattened into extremely thin sheets. However, even after filling with helium, the balloon just barely floated off the ground.
- Show students how to enter exponential numbers on their calculator. They usually believe that 5 x 103 is entered as 5, x, 10, EXP, 3, =.
- Treat all periodic properties as a consequence of Coulomb’s Law. Going across the table properties are a function of the charge and going down the table they are a function of size.
- Do your Bi 12 teachers a huge favour — teach your students how hydrogen–bonding works.
- Molecular mass is a superfluous topic. There is no need to teach it.
- Teach dilution by showing students how orange juice is diluted. All molarity concepts involving dilution can be taught in this intuitive manner which does not rely on using complicated equations.
- Students frequently confuse the details of exothermic and endothermic reactions. I have found the following to clear up almost all such confusion permanently. Exothermic is a “loser” (sorry for the political incorrectness.). Exothermic reactions give off heat (loser can’t even keep its own heat). The potential energy graph goes DOWN (loss). The value of ∆H is NEGATIVE. The surroundings get warm at the expense of the reacting chemicals. Heat is written on the products side (doesn’t want to be associated with the reactants). I know it’s a little silly, but students won’t forget these connected ideas and will remember them all through high school Chem.
- Occasionally a re-test is required. Sure, you taught well and you thought the kids were ready, but the results indicated they didn’t have a sufficiently good grasp of the material. I have found the following to be a very fair way of handling re-test marking; a way that students also agree is very fair. I make the re-test optional (say at lunch hour). To each student’s previous score add 75% of the improvement they make in their mark on the new test. Why? Well, if you give the better of the two marks, students get 100% of the improvement. The problem with this is that a student who received 95% on the first test and 95% on the second test feels a little cheated if another student’s mark goes from 45% to 99%. If you give the average of the two marks, students get 50% of the improvement. The problem with this is that a student with a very low mark, say 10%, who receives 80% on the re-test will have made an incredible gain and yet ends up with a demoralizing 45% at the end. But look at what giving 75% of the improvement does in the previous two cases. The student who went from 45% to 99% ends up with 85.5% (which is an “A”) and does not cause ill feelings from the student who scored consistently high on both tests. In the second case, students who went from 10% to 80% receive a final score of 62.5%, which is a “C”, and feel their renewed efforts at learning have been rewarded. Incidentally, the easiest way to calculate the combined mark is:
- Students frequently don’t understand the significance of an empirical formula. Get students to work out the percentage composition of CH3 and C2H6 , and work backward to the empirical formula.
- Alchemists tried to convert base metals into gold (unsuccessfully). However, in 1980 scientists at Lawrence Labs (University of California at Berkely) were able to transmute a bismuth sample into one-billionth of a cent’s worth of gold using a particle accelerator for a cost of $10 000.
- Subscribe to Journal of Chemical Education, Chem 13 News, and every science-related journal you can lay your hands on. Then relate what you have read to students in the form of a “Useless fact for the day”. These “facts” convey the worth of science and make them think of science in ways other than what comes over in routine lessons. For example: gorillas spontaneously swore in sign language, earthworms in a maze learn just as quickly as human infants, spider webs have built-in bungee cords.
Chemistry 12
- Le Chatelier’s principle is easily taught by visualizing a large fish tank divided in the middle by a separator having a hole in the middle. Fill tank; levels are equal initially. Add or remove a reactant or product and water flows (equilibrium “shifts”) to compensate).
- Biology teachers frequently confuse students by stating something like “as ATP changes to ADP, a phosphate bond is broken, releasing the energy which powers the cell”. This leaves students with the erroneous idea that one breaks a bond and energy pours out, like breaking an egg pours out the contents. Please ask your Bi 12 teachers to refrain from this misleading statement by giving the complete idea: when the phosphate bond is broken, the phosphate group bonds strongly to another molecule and the energy given off by this bond formation is greater than the energy required to break the original phosphate bond in ATP.
- Use an analogy of shooting a marble up a slope to show how molecules slow down as they approach each other and gain the necessary potential energy (activation energy).
- Place a little lead(II) nitrate in a tube and heat to decompose chemical into red–brown NO2(g); seal tube. Use dry ice (or ice–salt mixture) and boiling water to show effect on the NO2 – N2O4 equilibrium.
- Use students to visually show effect on NO2 – N2O4 equilibrium when adding/removing heat. Students move from left to right of room, and vice versa, when you clap your hands. Students on N2O4 side can’t move unless you give them a gentle shove. Extra shoving produces more NO2 ; no push means N2O4 accumulates.
- Sometimes, non–tested stuff can lead to huge jumps in understanding. For example:
a) The forward rate being doubled causes the reverse rate to double at equilibrium.
Assume the height of the energy “hump” is lowered by an amount X. Then
and therefore both kf and kr are multiplied by the same value, exp(X/RT), and if this value represents a doubling of the value of kf then kr is doubled also.
b) Rate = k•[Reactant] shows effect of concentration on rate. “K” is “the nature of the reactant”.
c) At equilibrium, Rate(f) = Rate(r), so that: kf•[React] = kr•[Prod] which rearranges to:
and since kf and kr are constant for a react (if temp is unchanged) then ratio of [Prod]/[React] is unchanged. Since kf/kr is not equal to 1, in general, then in general [Prod] is not equal to [React].
- O2(g) <===> O2(aq) is an exothermic process, predicted by the fact that reactants are more random and the reaction must be an equilibrium. This explains why the intensely cold Japan Current, off the shore of B.C. Promotes such luxuriant marine growth and the South Seas are virtual deserts. Conclusion: the dissolving of all gases must be an exothermic process.
Give students specific instruction in how to rearrange and solve typical equations generated in equilibrium calculations. Their math is generally not up to the task.
- In a chain reaction we cannot uniquely define what is a reaction intermediate and what is a catalyst.
- Students need help in interpreting solubility diagrams: what it means to be above, on and below the solubility line, and what happens when temperature is changed.
- Students have a great deal of difficulty interpreting KE diagrams (# of particles versus KE). The problem is that they do not see that there are many “energy slots”: those at low energy are minimally occupied and those at high energy are minimally occupied while those in the middle are occupied by many molecules. Use an analogy of heights of 1000 babies (heights are crammed in a narrow range with relatively many in each height increment. By age 17, the 1000 students are distributed over a much wider range, with fewer students in each height increment. Age increase corresponds to a temperature increase.
- Government exams allot 2 marks to sig figs: one to a pH problem and the other randomly picked. Subtract up to 2 marks/ class exam for sig figs to force students to pay attention to sig figs. If you do not enforce sig figs on all calculations you are directly penalizing your students.
- Students have trouble understanding why some solubility product calculations involve the doubling of an ion concentration, some a squaring and some both a doubling and squaring. They must see that doubling an ion concentration or not depends solely on the source of the ions. Squaring a concentration depends solely on the equation involved in forming a precipitate.
- Students get confused with common ion effect problems, frequently forcing the equilibrium the wrong way when a common ion is added to a solution.
- Insist that students use ion charges correctly from the first day. Their success in Unit V depends on their strict attention to use of charges.
- Stress the recognition of amphiprotic ions in Unit IV. Many types of problems depend on quick recognition of the presence of an amphiprotic species.
- Students should memorize the common spectator ions. They will save a great deal of time by doing so.
- Troublespots: with government exam booklet, students frequently say ammonia is a strong acid. They also frequently pick the wrong Ka value when calculating the Kb value for an amphiprotic species.
- Students have difficulty with the levelling effect. It helps if they understand that the effect is true for strong acids and for strong bases and that the levelling effect is general for a solvent which auto–ionizes. For example: Compare 2 H2O(l) <===> H3O+ + OH– and 2 NH3(l) <===> NH4+ + NH2–
- If water is the solvent then strong acids simply produce solutions of H3O+ and a negative ion, while strong bases produce solutions of OH– and a positive ion. Similarly, if the solvent is liquid ammonia, a strong acid produces solutions of NH4+ and a negative ion, while a strong base produces solutions of NH2– and a positive ion.
- Frequently used trick question on government exam: “In the reaction A <===> B + heat, should you use high or low temperature to produce B at the faster rate?” Students confuse the effects of temperature on a reaction rate with the effects of temperature on equilibrium. They must be trained to identify the “key words” in such problems.
- There is no need to teach balancing of redox equation by both the half cell and oxidation number method. Most students prefer the method of half cells (and half cells will work for all nasty problems introduced at university level when the oxidation number method quickly fails). For a small subset of problems in which it is only necessary to know if oxidation or reduction has occurred, oxidation numbers are quicker.
B. Labs
Chemistry 11
- Spectrophotometry – it is cheaper and easier to use copper sulphate than cobalt nitrate – read the copper(II) absorption at 660 nm.
- The freezing point of a solid is easy to demonstrate (for example, let paradichlorobenzene cool in air in its test tube with a thermometer showing a drop, then a levelling and then a drop as the liquid solidifies. However, the melting point is much more difficult to see because of the small heat of fusion of paradichlorobenzene. The lack of a clear “levelling off” is confusing to students. Be careful! Personally, I feel a lab spent measuring the freezing and melting behaviour is not worth the time spent. A better lab is measuring the melting temperature of a substance using the capillary tube method. They could attempt to identify an unknown by its MP, possibly using some other physical properties in addition.
- Have students write out how they will find the solubility of saturated sodium chloride, expressed in grams of salt per 100 g of water. When they present a satisfactory plan, let them try it out with a sample of saturated salt solution.
Chemistry 12
- Students frequently find that when they make up solutions and titrate them the titration volumes required either steadily increase or steadily decrease. Show students that a steady increase is a result of using a wet pipette or improperly stirred stock solution being pipetted from top of stock bottle. A steady decrease means the burette was wet or improperly stirred stock solution was poured into burette or was pipetted from bottom of bottle.
C. Demonstrations
Chemistry 11
- Demonstrate that glass is soluble in water: Place a piece of soda glass tubing in phenolphthalein solution– no change in colour. Use a mortar and pestle to powder a small piece of soda glass tubing (NOT PYREX!). Add a few drops of phenolphthalein solution to the powder – it turns bright pink, showing that sufficient sodium carbonate has dissolved to turn the phenolphthalein basic.
- Demonstrate a synthesis reaction: Carefully stir together equal volumes of powdered aluminum and powdered iodine in a ceramic evaporating dish on several ceramic pads in a fume hood. Add two or three drops of water and wait. After a minute, a purple gas is given off, followed by flames. (A fire which starts with water!) The white solid formed is aluminum iodide. The heat is due to the rapid oxidation of aluminum by water (surface area effect). Application: During the Falklands Island conflict between Britain and Argentina, a British ship was hit by an Exocet missile, resulting in a small fire. When sea water was poured on the blazing metal, the fire exploded and the ship was lost. Subsequently, Britain and the US replaced the aluminum superstructures on many warships.
- Demonstrate a decomposition reaction: sugar + concentrated sulphuric acid. Do in fume hood. The surface area of the activated charcoal formed is about the area of one football field per gram. That’s why charcoal filters in cigarettes use just enough charcoal to bring tar levels down to “maximum allowable levels.” (If pure charcoal was used, nothing would get through the filter.)
- Demonstrate solvent extraction with 1% aqueous methylene blue extracted into butanol. The colour moves from water to butanol, and after 2 extractions about 99% of colour is gone from water.
- Use a soft pencil to blacked one side of an index card and then use a hole–punch to punch paper circles out of the index card. Have a 250 mL flask, with stopper that has a layer of water on bottom (75 mL covered by about 75 mL of petroleum ether or similar non–polar solvent). Put a couple of dozen paper circles in the mixture and announce to the class that you are proposing a bet. You will shake the flask and if more circles come white face up then everyone gets an extra 10% on the next test; if more black faces come up, they lose 10%. Shake. (Almost every one comes up black!)
- Demonstrate halogen reactivity by sprinkling powdered antimony into three large graduated cylinders containing chlorine, bromine and iodine.
- Demonstrate molar mass by sealing contains with one mole of water, sucrose, ethanol, aluminum, iron, copper(II) sulphate, etc.
- Demonstrate that saturation is a relative concept by adding potassium permanganate to saturated sodium chloride. The solution is saturated with respect to salt but not potassium permanganate.
- Demonstrate that if the “schlieren effect” (heat wave appearance) is seen, a solution having a solid at the bottom is not yet saturated. This is a quick test (and approximate) for saturation.
Chemistry 12
- “Grecian formula” men’s hair colouring contains lead(II) acetate! The lead ion reacts with sulphide linkages in hair, depositing a layer of lead sulphide (galena) on the hair shaft and darkening the hair. Addition of iodide ion to Grecian Formula causes a yellow precipitate of lead(II) iodide.
- Students get confused by the concept of potential energy and kinetic energy. For example, they frequently believe that substances having a high potential energy must be hot. To show the difference in a strikingly dramatic way do this demo. Prepare a set of 18 x 150 mm test tubes containing melted sodium thiosulphate as follows. Fill the test tube with sodium thiosulphate pentahydrate crystals and add about 2-3 mL of distilled water. Carefully melt the contents, using a stirring rod to ensure even heating, wipe the lip of the tube with a wet paper towel, seal with a rubber stopper (not a cork) and allow to cool overnight. Give test tubes to students (make sure they don’t jiggle or tip them), have them note the temperature of the tube, and then simultaneously remove the stopper and add a small crystal of sodium thiosulphate. The contents quickly crystallize and give off heat. Point out that the energy given off was put in the previous day and was present as stored/potential energy before being converted back to kinetic energy. Also, cold nitroglycerine has a huge potential energy.
- Heat up a 25 x 200 mm test tube containing about 25 mL of saturated calcium acetate. Reverse solubility allows solid to form as solution is heat; redissolves when cooled.
- Demonstrate that cereals contain reduced (elemental) iron by grinding Cheerios, say, in a mortar and pestle, putting powder into a beaker of water on a magnetic stirrer and collecting the iron off the magnet after a minute if stirring.
- To demonstrate an entropy–driven reaction, take a small wooden block (mine is 1” x 3” x 4” and smooth), pour water over it and place a 250 mL beaker on the block. Into beaker, pour about 25 g of NH4SCN and 25 g of Ba(OH)2•8H2O and stir. Ammonia gas is given off and the solids appear (to students) to melt. After one minute, pick up the beaker and the wood block comes with it because the mixture’s temperature has fallen dramatically.
- Add concentrated HCl to some concentrated NaCl and ask students to explain the presence of the resulting precipitate. If written as a double replacement equation, the reaction puzzles students:
- Demonstrate the Ka values of indicators by adding indicators to solutions having pH 3 to pH 11
- Demonstrate rapid corrosion by adding a drop of saturated mercury(II) nitrate on top of a sheet of polished aluminum and scratching the mercury compound into the aluminum surface. After about 15 minutes a visible pile of aluminum oxide will be pushed up from the scratches by the mercury(II) ion.
D. Equipment, Chemicals and Resources
- Use aspirator pumps to improve filtration speed.
- Use 4 inch diameter black PVC pipe as splash guards for aspirator pumps.
- Have at least 4 L of saturated NaCl on hand. It constantly comes in handy.
- Have at least 250 mL of saturated calcium acetate on hand.
- Use small 1/4” PVC bends for connecting two vacuum flasks.
- Use 9 cm Buchner funnels to improve the speed of filtration. Beware of the cheap ones from Northwest Scientific; the filter paper easily gets holes blown through them, even with doubled filter paper.
- When growing crystals, tie the “seed crystals” on with invisible mending thread (available from sewing stores). File a small notch in a seed crystal to hold the thread on better; the notch does not affect the crystal’s growth.
- Neutralizing solution = 87.2 g anhydrous sodium acetate + 5.7 mL conc acetic acid/1 L
- Cornstarch packing pellets (used to pack orders from Boreal Labs) are excellent sources of instant starch; just pop a pellet or two into boiling water and get a starch solution in minutes,
- Flatten styrofoam balls and glue on small squares of plastic magnet strip. Use various sizes of balls and spray paint different colours. Make molecules and crystals by sticking the magnetized balls to the blackboard (unfortunately, not all boards) or a big piece of galvanized sheet.
- Make your favourite molecules out of coloured styrofoam balls: H2O, NH3 , CH4 , CH3CH3 , etc. This is a great project for students.
- Keep extra 0.1 and 1 M HCl and NaOH on hand for spontaneous demos
- keep buffers on hand, especially pH 4, 7 and 10
- Buy 35 & 50 cc plastic syringes from a drugstore – buy lots and use them for pipette pumps
- Keep Alka Seltzer on hand. Very useful for both Chem 11 and 12 (especially 12)
- Show students how to stir the contents of a test tube which is no more than 20% full: tap the bottom of the tube several times, from the side.
- Show students how to read a burette properly. Place a finger behind burette, just below meniscus or place a horizontal black line on a piece of card stock behind and below the meniscus. The true bottom of the meniscus is hard to see (and universities typically require a reproducibility of ±0.01 mL).
- Keep a special “demo drawer” containing the special equipment you have made or assembled. Then, when you need to do a particular demo on the spur of the moment, “the drawer is ready”.
- Instead of using powdered manganese(IV) oxide when generating oxygen, use solid MnO2 in the form of the mineral pyrolusite, available cheaply from
- D J Mineral Company, P.O. Box 761, Butte, Montana 59703-0761, Phone: 406-782-7339, Fax: 406-494-2455 (Cost is about $2/ lb; 5 lb is more than enough for a lifetime)
The pyrolusite slows the reaction to a reasonable rate and is reusable indefinitely.