Carbohydrates, Proteins, Vitamins and Minerals

|
Carbohydrates, Proteins, Vitamins and Minerals |
By Dr. Mohd. Amirul Islam
Structure
- Introduction
- Learning Objectives
- Carbohydrates
- Sources of Carbohydrates
- Structure of Carbohydrates
- Classification of Carbohydrates
- Importance of Carbohydrates
- Functions of Carbohydrates
- Carbohydrates Deficiency Diseases
- Proteins
- Sources of Proteins
- Structure of Proteins
- Classification of Proteins
- Importance of Proteins
- Functions of Proteins
- Proteins Deficiency Diseases
- Vitamins
- Classification of Vitamins
- Vitamin A (Ratinol)
- Vitamins D (Cholecalciferol)
- Vitamin C (Ascorbic acid)
- Vitamin B1 (Thiamine)
- Vitamin B2 (Riboflavin)
- Minerals
- Kinds of Minerals
- Sources and Functions of Minerals
- Mineral Deficiency Diseases
- Let's Sum Up
- Key Points
- Glossary
- Practice test
- Answer to SAQs
- References and Further Readings

|
Introduction |
There are seven main classes of nutrients that the body needs. These are carbohydrates, proteins, fats, vitamins, minerals, fiber and water. It is important to consume these seven nutrients on a daily basis to build and maintain health. Deficiencies, excesses and imbalances in diet can produce negative impacts on health, which may lead to diseases such as cardiovascular disease, diabetes, scurvy, obesity, or osteoporosis as well as psychological and behavioral problems. According to the reports of the United Nations World Health Organization (WHO: 1996), more than starvation the real challenge in developing nations today is malnutrition-the deficiency of micronutrients (vitamins, minerals and essential amino acids) that no longer allows the body to ensure growth and maintain its vital functions. We will discus about the sources, classification, importance and deficiency diseases of carbohydrates, proteins, vitamins and minerals in this unit.
| Carbohydrates |
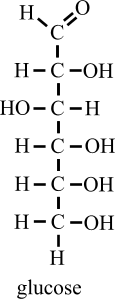
Carbohydrates are the polyhydroxy organic compounds made up of carbon, hydrogen and oxygen in which the ratio of hydrogen and oxygen hydrogen is 2:1 exactly as H2O (2:1).
(![]() : Can you add some more sentences to this part?)
: Can you add some more sentences to this part?)
| Sources of Carbohydrates |
The main sources of carbohydrates are plants, e.g., starch (storage forms carbohydrate of chlorophyll containing plants), sugars, cereals, potatoes, legumes, millets, roots and other vegetables. Sugars are found in fruits, juice, cane, honey, palm, milk, etc.
(![]() : Can you add some more sentences to this part?)
: Can you add some more sentences to this part?)
angular cheilitis see cheilitis treatment cheilitis treatment chelitis chelitis
| Structure of Carbohydrates |
- Chemically, they are aldehyde or ketone derivatives of higher polyhydric alcohol (having more than one "OH" group). They may be identified by the type and number of monosaccharide residues (glucose/ fructose molecule) in their molecules. The general formula of carbohydrate is Cn (H2O)n where n=3-9.
- Each sugar molecule consists of a backbone of carbon atoms linked together in a linear array by single bonds. Each carbon atom is linked to a single hydroxyl group except for one that bears a carbonyl (C=O) group. If the carbonyl group is located at an internal position, the sugar is a ketose (e.g., fructose). If the carbonyl is located at one end of the sugar, it forms an aldehyde group or aldose (e.g., glucose).
| Classification of Carbohydrates |
Carbohydrates may be classified into the following four major groups -
- Monosaccharides: Monosaccharides are the simplest form of carbohydrates. All carbohydrates are reduced to this state before absorption and utilization. They contain three to six carbon atoms. General formula is Cn(H2O)n.
- Disaccharide: Disaccharides Consist of two covalently joined monosaccharide units. They are produced as two molecules of the same or different monosaccharides on hydrolysis. General formula is Cn(H2O)n-1, e.g., lactose, sucrose, maltose etc.
- Oligosaccaharides: Oligosaccaharides consist of few number (2-6) of monosaccharide units e.g., glycoproteins.
- Polysaccharides: Polysaccharides are composed of many molecules of monosaccharides linked together. General formula is (C6 H10 O5)x. e.g., Glycerole.
| Importance of Carbohydrates |
- Carbohydrates, such as energy yielding compounds D-Ribose, are the structural elements of nucleic acid and coenzymes.
- Act as intermediates in hexose monophosphate stant.
- D-Lyxose, a constituent of a lyxoflavin isolated from human muscle.
- D-glucose carried out by the blood and used in tissues.
- D-fructose can be changed to glucose in the liver and intestine and used in the body.
- Glycosides are important in medicine.
- Hexosamines is used as antibiotic.
- Monosaccharides are important constitute of nucleotides and nucleic acids.
- Disaccharides act as an intermediate in the digestion, important as a dietary constituent and major source of energy in the diet.
- Starch and glycogen serve as temporary stores of glucose in plants and animals respectively.
| Functions of Carbohydrates |
- Glucose act as energy yielding compounds, the major fuel of the tissue, constitutes the structural material of the organism, converted to other carbohydrates having highly specific functions.
- Glycogen acts as important storage of food material of the organism.
- Play a key role in the metabolism of aminoacids and fatty acids.
- Act as protective function-mucosubstance.
- Act as intermediates in respiration and carbohydrates metabolism e.g., (trioses).
- Participate in lipid synthesis.
- Pentoses - Synthesis of nucleic acid; Some co-enzymes (e.g., NAD, FAD, FMN, etc.); ATP, ADP, AMP, and also synthesis of polysaccharides.
| Carbohydrates Deficiency Diseases |
- Hyperglycemia
- Glycosuria
- Galactosemia
- Pentosuria
- Diarrhoea and flatulence
- Ketone
- Under weight.
| Proteins |
Proteins are complex organic compounds. They are macromolecules or bio molecules composed of amino acids linked by peptide bond. The constituent elements of proteins are carbon (54%), hydrogen (7%), nitrogen (16%), oxygen (22%) and some may contain sulpher (1%) or phosphorus (0.6%). They are macromolecules of high MW and consisting of chains of amino acids e.g., hemoglobin, albumin, globulin, enzymes, etc.
| Sources of Proteins |
Peas, beans, poultry, cereals, lentils, milk, cheese, eggs, meat, wet and dry fishes, pulses, and nuts.
(![]() : Can you add little more in this sub-section?)
: Can you add little more in this sub-section?)
| Structure of Protein |
The basic unit of the protein molecule is amino acids .The protein molecules are composed of the union of a large number of amino acids. There are over 10,000 proteins in the human body. They all composed of different arrangements of the main 20 fundamental amino acids. The sequence of amino acids in each protein is specific and is genetically controlled by the DNA of the cell.
Chemical Structure
The synthesis of protein molecule takes place by the union of the-NH2 group of one amino acid with the-COOH group of another. Elimination of water is known as condensation and the linkage (bond) formed is a covalent carbon-nitrogen bond, called a peptide bond. The remaining part of amino acid is known as R group or side chain. In this way dipeptide or polypeptide is formed.
CH2-CH.NH2 + HNH.CH2COOH H2N-CH.CH2-CO-NH.CH2COOH+H2O COOH Amino group Carboxyl group
Fig. 2: General formula of an amino acid.
According to the modern views, the structure of protein is considered by several level of organization.
1. Primary level - peptide bond is formed by the amino acids. They are linked by carboxyl group of one amino acid with the -amion group of another amino acid through disulphide bonds and other covalent modification.
O O O
HN – CH – C – NH – CH – C – NH – CH – C ----
R1 R2 R3
Fig. 3: Primary structure.
2. Secondary level - peptide bonds are folded which indicates a coiled structure (e.g., globular proteins). In this folding the carboxyl and amino groups of the peptide chains are linked by hydrogen and disulfided bonds. Such folding is known as the secondary structure of the protein.
3. Tertiary structure - when the globular protein consists of a series of single helix. These models will have elongated structures with a larger axial ratio (length: breadth). The structure in their dimensions is maintained by covalent or other bonds and described as tertiary structure.
4. Quaternary structure - In this structure, there are several monomer units, each with appropriate primary, secondary and tertiary structures may combine through non-covalent interactions e.g., hemoglobin contains four subunits identical in pairs.
| Classification of Proteins |
Proteins may be classified in the following ways -
According to Structure
- Fibrous type with elongate molecule e.g., keratin
- Pounded type with globular molecule
- Intermediate type.
According to Composition
- Simple proteins -e.g., albumins, globulins, histones etc.
- Conjugated proteins - e.g., nucleoproteins, lipoproteins, chromoprotiens, flavoproteins.
- Derived protein e.g., metproteins, peptones.
According to function
- Structural type - eg., collagen, -keratin ,mucoproteins.
- Enzymes type - eg. trypsinase, carbonylase, glutaminase.
- Hormones type-e.g., insulin, glucagon.
- Transplant type - e.g., hemoglobin, serum albumin.
- Protective type - e.g., antibodies, thrombin, fibrinogen.
- Contractile type - e.g., myosin, actin.
- Storage type - e.g., casein, ovalbumin.
- Toxins type - e.g., diphtheria toxin, snake venom.
| Properties of Proteins |
- Colloidal or crystallized in nature.
- Soluble in water, weak salts solution and dilute acids.
- Each protein possesses a specific isoelectric point at which it is precipitated.
- Optically active.
- Most of the proteins undergo coagulation by heat or acid.
- Proteins undergo denaturation by many kinds of chemical or physical treatment such as * shaking, change of temperature, change of reaction, additional of neutral salts etc.
- They differ from one another in chemical structure, physical and physiological properties.
| Function of Proteins |
- Proteins as enzymes - accelerate the rate of metabolic reactions.
- As structural cables - provide mechanical support both within cells and outside.
- As hormones, growth factors - perform regulatory functions and gene activators.
- As hormone receptors and transporters-determine what a cell reacts to and what types of *substance enter or leave the cell.
- As contract element -form the machinery for biological movements.
- Others - act as the defense against infections by protein antibodies, service as toxins, form blood clots through thrombin, fibrinogen and other protein factors, absorb or refract light and transport substances from one part of the body to another.
- Constitute about half of the dry weight of most organisms and maintain growth.
- Maintain colloidal osmotic pressure of blood.
- Act as acid base balance.
- They perform hereditary transmission by nucleoproteins of the cell nucleus.
- Most fibrous protein plays structural roles in skin, connective tissue of fibers such as hair, silk or wool.
| Protein Deficiency Diseases |
- Abdominal enlargement, excessive loss in urine and disease to lower urinary tracts-
- Vomiting
- Diarrhea
- Nephrosis
- Lassitude
- Oedema
- Kwashiorkor (Protein malnutrition)
- Marasmic - Kwashiorkor
- Negative nitrogen balance.
| Vitamins |
Vitamins may be defined as organic compounds occurring in small quantities in different natural foods and necessary for the growth and maintenance of good health in human beings and certain experimental animals.
They cannot be synthesized in the body but supplied by the diet to the human body. Plants produce all vitamins but animal (human) stores them. Some are produced in the body e.g. Provitamin carotene is converted into vitain A in the body and Vit. D is produced in the body in presence of ultraviolet radiation.
| Classification of Vitamins |
Vitamins are classified into two groups.
1. Fat - soluble vitamins
Fat-soluble vitamins are soluble in fats and fat solvents. They are insoluble in water. So these are utilized only if there is enough fat in the body e.g., vitamin A, D, E and K.
2. Water -soluble vitamins
Water-soluble vitamins are (heterogeneous group) soluble in water and so they cannot be stored in the body. 11 types of vitamins are included in this class e.g., thiamine, riboflavin, pyridoxine, cyanoccobalamine, niacin, pantothenic acid, biotin, folic acid and ascorbic acid, para-amino benzoic acid, and choline.
| Vitamin A (Ratinol) |
Properties
- Soluble in fat solvents and insoluble in water
- Viscous, colorless oil or pale yellowish substance
- Heat stable in absence of air
- Destroyed on exposure to air or ultra-violet rays.
Source of Vitamin A
Liver, heart, kidney, milk, codliver oils, fishliver-oils, butter, eggs, carrots, cabbage, vegetables, green leaves, mangoes, potatoes tomatoes, spinach, papaya etc.
Functions
- Effect on reproductive processes, differentiation, and immune system
- Essential for growth and night vision
- Helps in the preservations of structural and normal permeability of membranes, cell, * astrointestinal tract etc.
- Required for bone and teeth formation, influence genetic expression, reproduction to manufacture R.B.C etc.
- Maintain the health and activity of epithelial tissues, and glands prevent infection, maintains nutrition and function of the nervous tissue.
- Controls the action of bone cells and formation, helps in normal fertility and glucose synthesis.
- Acts as antioxidant.
- Helps in RNA and protein metabolism.
Vitamin A Deficiency Diseases
- Night-blindness, Xerophthalmia, Keratinisation of skin and mucous membrane.
- Retardation of growth in children, defective growth of bone and teeth, skin lesions, Bitot's, sports etc.
- Abnormalities in respiratory, GU and GI epithelium, Diarrhoea, Kidney stone, bladder disorders, infections of vagina, depression of immune reactions, anaemia, injury to brain and nerve causes paralysis, stunted skull and spine.
| Vitamins D (Cholecalciferol) |
Properties
- Soluble in fat solvents but insoluble in water
- Heat stable
- White crystalline material
- Ordinary boiling does not destroy it.
Source Fish liver oils e.g., cod liver oil, halibut - liver oil etc. Butter, milk, eggs, liver. In sub coetaneous tissue, 7 dihydrocholesterol is conveted to vitamin D by UV light.
Functions
- Control calcium and phosphorus absorption from the small intestine, concerned with calcium metabolism, helps in the bone and teeth formation.
- Minimize the losses of calcium and increases phoshate excretion by the kidneys, affects insulin secretion in pancreases.
Deficiency Disease
Causes Rickets (directive bone growth) in childless, osteomalacia in adults, disturbs calcium and phosphorous absorption.
| Vitamin C (Ascorbic acid) |
Properties
- White crystals, soluble in water, heat lavish
- Good reducing agents
- Early oxidized at 1000C in presence of oxygen
- Cannot stand cooking or canning
Sources
Guava, amla, green chilli, amaranth leaves, citrus fruits, green vegetables, potatoes, tomatoes, cheese, milk etc.
Functions
- Acts as antioxidant.
- Essential for formation of collagen present between cells.
- Necessary for the formation of osteoblasts and red blood cells.
- Helps to reduce the ferric iron (Fe3+) to ferrous iron (Fe2+) and is absorbed only in this form.
- Essential for the utilization of folic acid
- Takes part in oxidation and reduction reactions in the tissues.
- Helps in bone formation.
- Helps in wound healing.
- Prevents formation of free radical in the body.
Deficiency Diseases
- Scurvy, a disease characterized by sore, spongy gums, loose teeth, fragile blood vessels, swollen joints, and anemia.
- Delay in wound healing.
- Pain in bones.
- Skin becomes rough and dry.
- Pyrexia, rapid pulse and susceptibility to infection.
| Vitamin B1 (Thiamine) |
Properties
- White, crystalline substance
- Water-soluble
- Heat labile
- Unstable at high temperature and in alkaline medium
- Stable in acid medium
- On oxidation it gives a yellowish dye called thiochrome.
Sources
Rice polishing, dried yeast and wheat germ are rich sources of vit. B1. Whole cereals like wheat, oats, legumes, oil seeds and nuts are good sources. Milled cereals, vegetables, fruits, meat and fish are poor sources. On milling, vit. B1 is lost from cereals.
Functions
- Acts as a co-enzyme in carbohydrate metabolism
- Require for the synthesis of glycine
- It has a specific action on nerve tissue
- Requires for the maintenance of normal gastro-intestinal tone and motility
- Maintains normal appetite.
Deficiency Diseases
- Beriberi - nervous, system affected, muscles become weak and painful paralysis can occur.
- Heart failure, wet beriberi, dry beriberi, infantile beriberi, oedemia, children's growth is impaired, keto acids accumulate in the blood, wernicke’s-korsakoff’s syndrome etc.
- Loss of appetite, fatigue, irritability, depression and constipation occur.
| VitaminB2 (Riboflavin) |
Properties
- Yellow crystals
- Soluble in water
- Heat soluble in neutral and acid media
- Destroy by light.
Sources
Milk, liver, kidney, muscle, butter, chicken, fish, yeast, cheese, raw egg, white grains, green vegetable such as spinach, peanuts, fruits such as apple, orange etc. Functions
- Precursor of coenzymes (FMN and FAD) in oxidation-reduction reactions of electron transport chain, fatty acid synthesis etc.
- Essential for growth, essential for tissue oxidation related to carbohydrate, fat and protein metabolism.
- Maintain mucosal, epithelial and ocular tissues.
- Essential for normal vision.
Deficiency Diseases
Symptoms
- Tongue sore at the corner of the mouth.
- Loss of hair, skin becomes dry and scaly.
- Arrest of growth.
- Dermatitis around nose and lips, inflammation of tongue, angular stomatitis and cheilosis, photophobia, cataract etc.
- Scrotal or vulval dermatitis, intense itching etc.
- Disturb carbohydrate metabolism.
| Minerals |
(![]() : Check this part, particularly the gm per day)
: Check this part, particularly the gm per day)
Minerals are inorganic substances that serve a variety of functions such as cofactors in enzyme-catalyzed reactions, in the regulation of acid-base balance, in nerve conduction and muscle irritability, and as structural elements in the body. Each mineral is required in specific amounts ranging from y to gm per day. Some of the more important of these are calcium, phosphorus, sodium, potassium and iron.
| Kinds of Minerals |
Minerals may be divided arbitrarily into 2 groups.
- Macro minerals: The minerals, which are required in amounts greater than 100 mg/ day.
- Micro minerals: The minerals, which are required in amounts less than 100 mg/ day.
| Source and Function of Minerals |
Macro Minerals
Source and Function
- Calcium: Milk, egg, leafy green vegetable, fish, meat soybeans etc. Formation of bones and teeth structure. Activates ATP during muscular contraction, helps in blood clotting and capillary permeability.
- Phosphorus: Milk, peas, meat, fish, eggs, cottage, cheese, almonds, wheat germ, soybeans, black beans etc. Synthesis of nucleic acid, ATP and some protein. Helps in calcification of bones, maintain buffer system in body and bone formation.
- Potassium: Spinach, butter, beans, oranges, milk, peas, meat, fruits nuts, and vegetables. Involves transmission of nervous impulses chemical reactions and acid base balance in the body.
- Sodium: Table salt, eggs. meat, milk, cheese, butter, margarine, bacon etc. Form part of tissue fluids inducing blood, involves kidney functioning and transmission of nervous impulses, acid-base balance in body.
- Sulfur: Protein e.g., meat, fish and milk,
- Synthesis of proteins e.g., Keratin and many other organic confounds e.g., coenzymes A.
- Manganese: Vegetables and most other foods
- Constituent of bones and tooth structure, co-factor for many enzymes e.g., ATP-ase.
Micro minerals
Source and Functions
- Iron: Liver, eggs, meat, dark and green vegetables, lentis, potatoes, soybeans, chick peas, black beans, spinach, etc. Forms part of haemoglobin, helps in electron transport in biochemical reactions.
- Fluorine: Water, milk etc. Needed for strong enamel on teeth, as calcium deposit in bone
- Nitrogen: Protein e.g., meat, fish and milk,Synthesis of protein NA and many other organic compounds, e.g., coenzymes and chlorophyll
- Manganese: Vegetables and most other foods; Bone development (a growth factor)
- Cobalt: Liver and red meat; Red bloods cell development
- Copper: Most foods; Melanin production
- Zinc: Most foods; CO2 transport in vertebrate blood
- Molybdenum: Most foods; Hydrolysis of peptide bonds in protein digestion
- Boron: Most foods; Reduction of nitrate to nitrite during amino acid synthesis in plants.
- Iodine: Seafood’s, such as fish, shellfish and fish oil. Vegetables, spinach, fruits, and cereals.
| Mineral Deficiency Diseases |
- Nitrogen-kwashiorkor
- Sodium-muscular cramps, giddiness, anorexia, scanty urine, dry mouth, inelastic skin and disorientation.
- Chlorine-muscular cramps, renal disease etc.
- Calcium-poor skeletal growth, rickets in children, osteomalacea in adults.
- Manganese-poor bone development
- Iron-anemia, weakness, lethargy, brittle nails, koilonychia, palpitations, breathlessness etc.
- Zinc-poor appetite, mental lethargy and delayed wound healing etc.
- Cobalt-pernicious anemia,
- Fluorine-dental caries
- Iodine-goitre, cretinism in children
- Potassium-muscular weakness, paralysis, mental confusion, loss of appetite, nausea, abdominal distension.
- Phosphorus-rickets in children, osteomalacia in adults.

|
Results |

|
Practice Test |
Short questions
1. What do you mean by carbohydrates?
2. What are the diseases caused in deficiency of Carbohydrates?
3. What do you mean by proteins?
4. What do you mean by vitamins?
5. What are the fat-soluble vitamins, water-soluble vitamins and where are they found?
6. What are the diseases created by the deficiency of vitamin A, C and B1?
7. What do you mean by minerals?
8. What do mean by macro and micro minerals?
Analytical Questions
1. Describe the structure of Carbohydrates.
2. Classify the Carbohydrates.
3. What are the properties, sources and functions of vitamin A, B1, and C?
4. Briefly describe properties, some functions and daily requirements of ascorbic acid and folic acid.
5. Discuss the diseases, which are occurred in mineral deficiency.