Water Treatment
This is based on New Zealand Qualification Authority Unit 8039 [1] entitled "Explain and demonstrate the role of microorganisms in water treatment."
Contents
1.1
Microbial populations of water contain bacteria,virus and other small organisms. Various chemical and physical factors may influence these populations. Sunlight increases the growth of bacteria and zooplankton. Temperature fluctuations increase bacterial growth sometimes causing infection developing.
Phototrophs are organisms which carry out photosynthesis by using energy from sunlight to convert carbon dioxide and water into organic material to be used for cellular functions. The main phototrophs found in aquatic habitats are algae such as kelp, protists like euglena and bacteria such as cyanobacteria. These use hydrogen from hydrogen sulphide instead of water for chemical purpose and absorb light source as ultra violet or infrared.Phototrophs can be photoautotrophs ,photoheterotrophs or photolitotrophic autotrophs. The latter use light energy and electron sources like water,hydrogen and hydrogen sulphide for photosynthesis.[2]
Bacteria living in water have a varying range of nutritional requirements. Phototrophic nutritional requirements are outlined at Bacterial Microorganisms Nutrition [http://www.microbiologyprocedure.com/bacterial-nutrition/phototrophs.htm. Phototrophs use energy from sunlight to get high energy electrons (attached to carriers high on redox tower). They use CO2 and Calvin-Benson cycle to make all organic molecules.
[[http://www.biologie.uni-hamburg.de/b-online/library/micro229/terry/229sp00/lectures/autotrophs.html. Light is trapped by a patch of pigments (antenna field), getting passed around to a reaction center where an electron is released from Mg++ ion with high energy. It is then passed to electron transport system. From this point of using electron transport systems it generates proton gradients to make ATP. It needs to make not only ATP(available from proton gradient), but also NADPH. There exist two solutions to this problem. First, by using a reduced molecule with high redox potential like hydrogen gas(H2) or, hydrogen sulfide(H2S) to pass electrons to NADP+. Light is not needed for this. Second, by using a reduced molecule (with low redox potential) like water to release electrons and H+ ions. Requiring lots of energy to drive this reaction, an extra step, including light, is needed.
Chemoautotrophy is the use of inorganic molecules as a source of energy and is unique to bacteria.Chemolithotrophs then are microbes using inorganic compounds as electron donors and rely on them for energy. The term implies they are 'rock-eaters'(litho-trophs). They exist on minerals in their surrounding habitat and utilise carbon dioxide as their carbon source. Examples include: Sulphur-oxidisers, Thiobacillus denitificans, Iron-oxidisers,Thiobacillus ferroxidans, and Ammonium/Nitrite- oxidisers, Nitrosomonas spp. or Nitrobacterspp.
This website explains Chemoautotrophic and Chemolithotrophic Bacteria: http://www.enotes.com/microbiology-encyclopedia/chemoautotrophic-chemolithotrophic-bacteria
1.2
The environmental factors in the water can also effect the microbial population. This website out lines some of the effects it can have Effects of Environmental Factors on Microbial Populations in Brackish Waters off the Southern Coast of Finland.
Water, as the major source for the Earth's biosystems, is in continuous circulation. The microbial population in any body of water, is dependent on the state of water, be it; atmospheric, surface or groundwater. Because each environ is so diverse, microbial populations; their composition, effects and actual counts are subject to the physical and chemical make-up along with seasonal changes experienced by that body of water.
[http:www.microbiologyprocedure.com/aquatic-environment-microbiology]
Water containing a significantly large amount of solutes may not be osmotically compatible or "available" for use by micro-organisms, so the concept of water availability needs to be considered.[3]
1.2.1
Temperature.As temperature rises micro-organisms generally grow faster (at an exponential rate), but above their optimal growth temperature level, they experience dehydration or lysis.
[http:www.doi.wiley.com/10.1002/jsfa.2740680114]
More than 90% of the marine environment is below 5oC- a condition favourable to psychrophilic microbes,"cold-lovers". They can sustain growth at 0oC and cannot stand temperatures above 20o.
Thermophiles are at the other end of the scale. Thermus aquaticus, can grow optimally at 70- 72o. It is a common inhabitant of thermal springs[http:www.serc.web123.no/Atlantic Reiser/uib/thermophiles2007/]
1.2.2
pH Or concentration of Hydrogen ions. Most often than not, bacteria grow best at a pH of 6-8, with pH 7 being neutral. Acidophiles enjoy habitats of pH 7 down to pH 1[4]Alkophiles thrive at pH ranging from 7 to 14 with new bacteria identified recently [5]
1.2.3
Pressure: As the water gets deeper, the pressure increases. There are micro organisms that can live deep in the water and grow under huge pressure. These organisms have a strong cell that doesn’t collapse under the strain. Any other micro organisms would either freeze by the drop in temperature or be crushed by the increased pressure. Studies of these organisms can be difficult as they have to be cultured under pressure and have to be sampled using a pressure steel bomb. Without it, the cells explode. Cells have to be incubated at -2°C and have a very slow growth rate. These micro organisms are described as barotolerant (can grow at a maximum 400atm), barophillic (grow at greater than 400atm) and Extreme barophillic (grow at pressures greater than 700atm and cannot grow at low pressures).
1.2.4
Salinity: Some micro organisms are sensitive to salt and are unable to control their osmotic activity if they are placed into water with high salinity. Other microbes survive well in high salinity. These particular microbes are described as halophiles.
[Power Point presentation - Microbial Populations in H2O: chemlithotrophs][6]
2.1 - 2.2
The following links show the U.S. drinking water standards[7] and WHO's drinking water standards[8]. The following web sites provide various water treatment methods, such as sedimentation, flotation, filtration, etc., including the history of water treatment[9][10]. Water supply systems in New Zealand are nationally managed by each districts Regional Councils. For Manawatu water quality consult[11]. Each Council installs and maintains water systems according to regulations of the Resource Management Act,1991 [12].
Drinking water identifies some sources of suitable drinking water. For water to meet NZ standards, the Drinking Water Standard (DWSNZ) last updated in December 2005. The significant change from the 2000 update was to move the already stringent, quality control measures toward a broader approach of quality assurance. This shift in focus is a result of improved scientific knowledge and an opportunity to address broader issues.
Potable water treatment outlines various treatment processes in the preparation of 'water able to be put in a pot' or simply, safe to drink. Modern facilities assume water available to be potable, unless publicly stated otherwise.
[Power Point presentation - How potable H2O is produced][13]
Water resources are any source of water which is either useful or available to humans. Agricultural, household, recreational are the main usage of water. 97.5% of water is in sea (saline), 2.5% water as fresh water. Two thirds of freash water is frozen as glaciers and polar ice caps[14]
3.1 - 3.2
Water treatment is a process of removing contaminants from waste water. There are physical, chemical and biological process. The purpose of waste water treatment is to produce water suitable for release to the environment. The three types of waste water treatment are primary, secondary and tertiary.
In primary treatment,the dissolved biological mass is converted to sold mass by micro organisms and then biological solids are neutralized. The treated water is disinfected by chemical and/or physical methods. Water is then returned into streams, rivers, or lagoons for drinking, industry, irrigation and agriculture purposes. Solid waste like sticks,cans etc., can be treated with the sedimentation technique. In these, sewage passes through primary sedimentation tanks.
Secondary treatment, is used for degrading biological content present in the sewage like linen waste,food waste ,soap and detergent. This is done by using aerobic biological process because oxygen is needed for the biodegradation. In these processes, micro-organisms like bacteria and protozoa digest soluble organic contaminants like sugar,fat, organic short chain carbon molecules. Activated sludge plants produce different mechanisms, that use dissolved oxygen. This increases the growth of bacteria which remove the contaminants.
Sedimentation is the physical water treatment for settling of suspended contaminents under the influence of gravity. It used as primary water treatment process. Filtration is the another water treatment process. It is used for the removal of suspended particles and unsettled floc. Most common filter system is sand filtering. It helps to remove organic compounds, produces sweet taste and reduces any odours.
Filter beds or oxidising beds:In older plants trickling filter beds used for settled sewage liquor to spread on the surface beds made up of coke,limestone chips etc. Liquor spread by radiating arms having a central pivot,biofilms such as bacteria,fungus and protozoa in the surface can reduce the organic contaminants.
Tertiary method is the final treatment which increase the quality before back to the environment(sea,river,lake,ground).Filtration:it reduce the remaining suspended matter for example filtration in activated charcoal remove toxins. Lagooning: Settlement and biological improvement through storage in man made lagoon and ponds. Constructed wetlands:methods having increased aerobic biological improvement ,called phytoremediation. Nutrient removal:waste water contains agricultural 'run-off' of nitrogen and phosphorus which increase algae and bacterial growth. the consequences result in the production of 'algal blooms' or eutrophication causing deoxygenation of water and also contamination from increased biomass. Methods for removing nitrogen(nitrification) and phosphorus(enhanced phosphorus removal) exist, the details of which can be found at [15]
The sludge collected in any sedimentation tank in the water treatment process has to be disposed of. In some instances the wet sludge is transported to the nearest sewage works where it is discharged into the raw sewage inlet channel. The presence of the added chemicals can help in the primary sedimentation of the crude sewage. Alternatively, the sludge can be sent to a landfill site after it has been concentrated into a cake by dewatering. The dewatering is carried out by pressure filtration, vacuum filtration or centrifugation. In pressure filtration, the sludge is pressed at high pressure between filter cloths. Vacuum filters are popular in the USA but few treatment works in the UK employ them. A slowly revolving drum, partly immersed in the sludge, carries a filter cloth through which water is sucked from the sludge under vacuum. In centrifuging, chemically or biologically conditioned sludge falls onto the centre of a rapidly rotating bowl. The solids are thrown to the outer edge of the bowl where they are removed by a scraper. With chemical-conditioned sludge, 80–98% of the solids can be separated this way. These solids would contain typically 10–35% water[16].
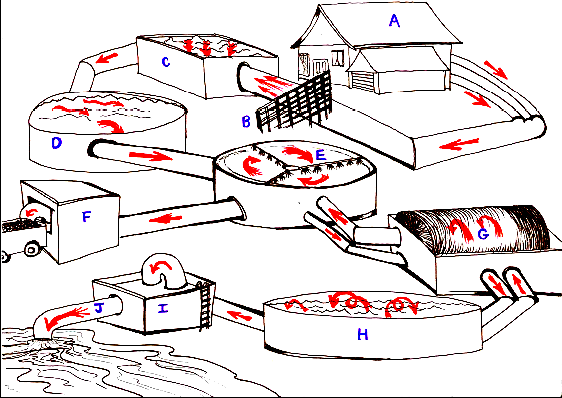
The diagram above offers a visual description of the pathway water might take through a sewage system. Not all treatment processes include the same steps, but all carry out removal, primary treatment, secondary treatment, quality control and eventual dispersion as adequately clean water.
A. Waste-water Removal. Pipes and culverts remove waste-water from domestic residences via a 'sewerage system'. Modern systems separate stormwater from other waste waters.
B. Preliminary Treatment Most devices here are designed to eliminate large debris, grease/oil, unsavoury rubbish. Consists of traps, screens or gridded bars.
Primary Treatment. C. Grit Chambers. Sewage is 99.9% water and 0.01% solids. The solids are organic, inorganic, suspended or dissolved. The sewage is pumped across chambers filled with grit and gravity pulls the smaller, dissolved particles toward the bottom.
D. Settling Tanks. Further separation of solids from effluent happens here in these settling tanks. In smaller communities or isolated homes, a septic tank provides primary treatment in the form of a settling tank. Large particles settle to the bottom where they are processed by anaerobic microbes. Effluent or dissolved particles move from the top, through to 'settle' here.
Secondary Treatment. E.Trickling Filters. Biological oxidising beds. Bring settled sewage into contact with microbial growth. Expensive to set up, they produce excellent results.
A stone filter medium provides ventilation to the beds base and plenty of places that micro-organisms can come into contact with the 'liquor' that continually flows past them. When the liquor reaches the bed it is pumped back to the top by aerated sprayers, rotating automatically.
F. Biosolid Removal. Settled organic solids and/or sloughed microbes can be removed here to reduce effluent contamination. Removal and further processing into fertilisers or organic mulch reduces the need to manufacture elemental fertilisers but is dependent on resources available.
G. Biological Contactor. Contactor circulates the bacterial media through the wastewater instead of circulating the wastewater over a fixed medium (as in the trickle filter system). The contactor has rotating discs containing bacterial slimes. As they rotate, one third is submerged and absorbs organic matter, while the rest is exposed to oxygen. Slimes build up and can be removed as in F.
H. Lagoons. Oxidation ponds or lagoons allow water to continually be subjected to light degradation of existing organics. Ponds are no deeper than 1.5m to allow light penetration. Consistently stabilises effluent, removing coliforms. Bacterial purification is related directly to the pond retention time.
Tertiary Treatment. I.Pump House A third step need to upgrade the quality of the effluent, dependent on intended use.
The water must be disinfected to ensure its quality. Disinfection is the inactivation of pathogenic organisms and is not to be confused with sterilisation, which is the destruction of all organisms. Worldwide, chlorine is the most popular disinfecting agent for drinking water, although the use of ozone has recently become more widespread. The use of chlorine in water treatment, while not being acceptable to all, does save lives (the water must be disinfected to ensure its quality). Disinfection is the inactivation of pathogenic organisms and is not to be confused with sterilisation, which is the destruction of all organisms. There is more details of the process of Disinfection
J. Effluent Emission CLEAN water is released into local streams or rivers. Continual public updates are provided for 'peace of mind'. Problems, queries and/or feedback can be targeted toward local councils.
[[17]]
Disinfection
[18]
Disinfection is a kind of waste water treatment used to reduce the number of microorganisms in water before it transported to environment. The methods commonly used for disinfection include ozone,chlorine,ultraviolet and chloramine. The effectiveness is dependent on the quality of water including aspects of clarity (cloudiness), and pH.
Chlorination It is commonly used in North America because of its high degree of effectiveness. One disadvantage is that it produces a chlorinated compound that is carcinogenic to the environment. Ultra violet light can be used instead of chemicals like chlorine and iodine. Because of lack of chemicals it does not cause harmful effect to microorganism but it can vary the genetic structure of bacteria causing an inability to reproduce. Other disadvantages are the high cost of lamp maintenance and replacement.
Ozone Is formed by passing oxygen through high voltage potential.It is unstable and oxidises all organic materials. It can be kept near the treatment site and is comparatively less poisonous than chlorine because it produces less by-products than chlorine.One disadvantage is found to be the high cost of equipment.[19]
This website has a slideshow that outlines Wastewater treatment.
[Power Point presentation - Treatment of Waste H2O][20]
5.1
In waste water treatment there are many ways to test water quality two such ways are BOD and COD. Wastewater parameters is a website that discusses the significance of this tests.
Biochemical Oxygen Demand(BOD) is a standardized means of estimating the degree of contamination of water supplies, especially those which may contain contamination from sewage, manufacturing processes or industrial wastes.
Chemical Oxygen Demand (COD) refers to the amount of oxygen, expressed in parts per million, that is consumed under specific conditions. This occurs in the oxidation of the organic and oxidizable inorganic matter contained in industrial waste waters.[21][22].