User:Sakshi grover/my-sandbox
Contents
- 1 SOLID STATE
- 2 Points to be verified through out this chapter
- 2.1 Classification of solids based on different bindind forces:molecular,ionic,covalent and metallic solids.
- 2.2 Amorphous and crystalline solids (elementary idea)
- 2.3 Unit cell in two dimensional and three dimensional lattices
- 2.4 Calculation of density of unit cell
- 2.5
- 2.6 Electrical and magnetic properties.
- 2.7 1.1 GENERAL CHARACTERSTICS OF SOLID STATE
- 2.8 1.2 CLASSFICATION OF SOLIDS
- 2.9 Difference between crystalline and Amorphous Solids
- 2.10 The crystalline solids have definite regular geometry because of orderly arrangement of constituents in three dimensional space
- 2.11 The crystalline solids have sharp melting points i.e.,they change abruptly into solid state at a fixed temperature.on the other hand amorphous solids do not have sharp melting points
- 2.12 The crystalline substance have physical properties such as electrical conductivity,thermal conductivity,mechanical strength,refrective index,etc.such are called ANISOTROPIC.
- 2.13 Amorphous substance have physical properties same in all direction and are called ISOTROPIC.
- 2.14 CRYSTAL LATTICE OR SPACE LATTICE AND UNIT CELL
- 2.15 A UNIT CELL IS
- 3 SEVEN CRYSTAL SYSYSTEM
- 3.1 1- Cubic.- All the three axes are equal of length and are at right angles to each other (a=b=c,all angles=90o)
- 3.2 2- Tetragonal.-The three axes are at right angles to each other but only the two axes are equal (a=b not =c all angles=90o)
- 3.3 3- Orthorhombic.- It has three unequal axes which are at right angles to each other (a not=b not=c.all angles=90o)
- 3.4 4- Monoclinic.- The three axes are of equal length length which are inclined at the same angle but angle is not equal to 90o.
- 3.5 5- Hexagonal.- It has two edges of equal length (a=b) abd two angles of 90o and one angle of 120o.
- 3.6 6- Rhombbohedral.- The three axes are of equal length which are inclined at the same angle but the angle is not equal to 90o.
- 3.7 7-Triclinic.The three axes are of equal length,and all angles are diffrent but none is perpendicular to any of theothers.
- 4 Three types of Cubic lattice
- 4.1 1- Simple or primitive. It has points at all the corners of the unit cell.
- 4.2 2- Body centered cubic unit cell (bcc). It has points at all the cornersas well as at the centre of the cube.
- 4.3 3-Face centered cubic unit cell (fcc)
- 4.4 TYPES OF CUBIC CRYSTALS AND NUMBER OF ATOMS PER UNIT CELL
- 4.4.1 The unit cell may be represented in three diffrent ways.
- 4.4.2 Simple or primitive cubic unit cell
- 4.4.3 Body centered cubic unit cell
- 4.4.4 Face centered cubic or cubic close packing unit cell.
- 4.4.4.1 (1)-- Simple or primitive cubic unit cell.
- 4.4.4.2 The number of atoms present in each unit cell = 8 corners X 1/8 atom per unit cell = 1 atom
- 4.4.4.3 (2)-- Body centred cubic unit cell.
- 4.4.4.4 The number of atoms present at the corners per unit cell = 8 corner atoms X 1/8 atom per unit cell= 1
- 4.4.4.5 Total number of atoms in bcc arrangement=1+1=2.
- 4.4.4.6 (3)-- The number of atoms present at corners unit cell = 8 corners atoms X 1/8 atoms per unit cell = 1
- 4.4.4.7 The number of atoms present at face unit cell = 6 atoms at the face X 1/2 atom per unit cell = 3
- 4.4.4.8 Total number of atoms in ccp or fcc arrangement = 1 + 3 = 4
- 5 STRUCTURES
- 6 INTERSTITIAL SITES OR INTERSTITIAL VOIDS
- 6.1 1- TETRAHEDRAL SITE OR VOID.-The vaccant space amoung four spheres having tetrahedral arrangement is called tetrahedral site or tetrahedral void.
- 6.2 2-Octahedral site or void.-The void formed by two equilateral triangles with apice opposite direction is called octahedral site or octahedra void.
- 6.3 PROPERTIES OF SOLIDS
SOLID STATE
As we look around,most of things we see are solid substance rather than liquids and gases.The solids are the substance which have definite volume and shape.Solids have regular order of their constituent particles(Atoms,molecules or ions.)These particles are held by strong forces,they present at fixed positions.The properties of the solid not only depend upon the nature of the constituents but also on their arrangements and the types of forces which hold the constituent particles together in tightly arrangement.
Points to be verified through out this chapter
Classification of solids based on different bindind forces:molecular,ionic,covalent and metallic solids.
Amorphous and crystalline solids (elementary idea)
Unit cell in two dimensional and three dimensional lattices
Calculation of density of unit cell
Packing in solids,voids,number of atoms per atoms per unit cell in a cubic unit cell
Electrical and magnetic properties.
1.1 GENERAL CHARACTERSTICS OF SOLID STATE
As We know that matter exist in three diffrent physical states namely SOLID,LIQUID and GAS.under a given set of conditions of temperature and pressure,the most stable state of substance depends upon the net effect of two opposing forces;intermolecular forces and thermal energy . ====
Characteristic properties of solids are: =
- Solids are rigid and have definite shape and mass.
- They have definite Volume irrespective of the size or shape of the container in which they are placed.
- The intermolecular distance in solids are short and intermolecular forces are strong.
- The constituent particles (atoms,ions or molecules)of solids have fixed positions and can only oscillate about theit mean positions.
- Solids are alomost incompressible.
- the densitry of solids is greater than that of liquid and gases.
- Solids diffuse very slowly as compared to liquid and gases.
1.2 CLASSFICATION OF SOLIDS
Solids can be classified on the basis of nature of order present in the arrangment of constituent particles in to two typesas:
- Crystalline solids
- Amorphous solids
1.CRYSTALLINE SOLIDS
The substance whose constituent particles are arranged in a definite geometric pattern are called CRYSTALLNE SOLIDS.
The Crystalline substance are said to have LONG RANGE ORDER.
2.AMORPHOUS SOLID
The substance whose constituent particles are not arranged in any regular arrangment are calledAMORPHOUS SOLIDS.
The amorphous solids are said to have SHORT RANGE ORDER.
Difference between crystalline and Amorphous Solids
:
1. Characteristics geometry
.
The crystalline solids have definite regular geometry because of orderly arrangement of constituents in three dimensional space
.
2.Melting points
.
The crystalline solids have sharp melting points i.e.,they change abruptly into solid state at a fixed temperature.on the other hand amorphous solids do not have sharp melting points
3.Isotropy and anisotropy
The crystalline substance have physical properties such as electrical conductivity,thermal conductivity,mechanical strength,refrective index,etc.such are called ANISOTROPIC.
Amorphous substance have physical properties same in all direction and are called ISOTROPIC.
4.Cleavage
Crystalline solids can be cleaved along definite planesWhen cut with a sharp edge tool.eg:- Knife
Uses of Amorphous Solids
- The most widley used amorphous solids are inorganic glasses.
- Amorphous silicon is best material for converting sunlight into electricity.
- Rubber is also a amorphous solid which is used in making tyres.
- Plastics which are amorphous are used in making article of daily use.
| S.no | Property | Cruystalline solids | Amorphous soids |
|---|---|---|---|
| 1. | Shape |
The crystalline solids have definite characterstic shapes. |
The amorphous solids have irregular shapes |
| 2. | Order in arrangement of constituent particles | They have regular arrangement of the constituent particles.They are said to exhibit long range order. | They do not have any regular arrangement of constituent particles.They may have short range order. |
| 3. | Melting point | They have sharp and characterstic melting point | They do not have sharp melting point.They gradually soften over a range of temperature. |
| 4. | Clevage Property | When cut with a sharp edge tool,they split into two pieces and the newly generated surfaces are plain and smooth. | When cut with a sharp edge tool they cut into pieces with irregular surfaces. |
| 5. | Enthalpy of fusion | They have a definite and characterstic enthalpy of fusion. | They do not have definite enthalpy of fusion. |
| 6. | Anisotropy | They are anistropic and have diffrent physical properties in diffrent directions. | They are isotropic and have same physical properties in all directions. |
| 7. | Nature | They are true solids. | They are pseudo solids and super cooled liquids. |
| 8. | Common Examples | Copper,silver,iron,common salt,Zince sulphide,potassium nitrate etc. | Glass,rubber,plastics,etc. |
CLASSIFICATION OF CRYSTALLINE SOLIDS
The Crystalline solids can be classified into following four types.depending upon nature of intermolecular forces.
- Molecular Solids
- Ionic Solids
- Metallic Solids
- Covalent or network solids
1.MOLECULAR SOLIDS
These are crystalline substance in which the constituent particles are molecules.
They are furthure divided in to 4 sub categories
Non polar molecular solids
- They are generally soft.
- They have low melting point and are in liquid or gaseous form.
- They are non conductors of electricity.
- They are volatile and have low enthalpies of vaporisation.
Polar molecular solids
These comprise of molecules of substances formed by polar covalent bonds.for example,solid HCL,solid SO2 etc.The molecules are held together by strong releatively stronger dipole-dipole forces.
Hydrogen bonded molecular solids.
The molecules of such solids contain hydrogen bonds between them.for example,Solid water(ice)
2.IONIC SOLIDS
These solids consists of positively and negatively charged ions arranged in a regular manner throughout the solid.ions are held together by strong coulombic(or electrostatic) forces.In ionic solids,the constituent particles are IONS.
Chracterstics of ionic crystals are:
- Ionic solids are very hard and brittle.
- They have very high melting points.
- They are poor conductors of electricity
- They have high enthalpies of vaporisation.
- They are soluble in water and also in other polar solvents.
Common examples of ionic crystals are:
Nacl,KNO3,LiF,Na2SO4 etc.
3. Metallic solids or crystals
In metalic crystals the constituent particles are positive ions.The electrons in metallic crystals are mobile and are evenly spread out throughout the crystal.Each metal atom contributes one or more electrons towards this sea of mobile electrons.
- Metallic crystals may be hard as well as soft.
- They are good conductors of heat and electricity.
- They have metallic lusture and color in certain cases.
- They are malleable and ductile.
- They have moderate enthalpies of fusion.
The examples of metallic crystals are Comon metals such as nickel,copper,alloys.
4. Covalent or Network solids or crystals
In covalent crystal,the constituent particles are Atoms which are linked together by a contiuous system of covalent bonds throughout the crystal.
They are also giant molecules.
- The covalent crystal are hard.
- They have extremely high melting points.
- They are poor conductors of electricity and are insulators.
- They have high enthalpies of fusion.
The examples are Diamond,Quartz,boron nitride (BN).
CRYSTAL LATTICE OR SPACE LATTICE AND UNIT CELL
A regular arrangement of the constituent particels (atoms,ions, or molecules) of a crystalline solids in three dimensional space.
A UNIT CELL IS
The smallest repeating unit in space lattice which when repeated over and over again produces the complete crystal lattice.
Parameters of a Unit cell
A unit cell is characterized by
- Its dimension along the three edges as a,b, and c.
- Angles,Alpha,Beta,Gamma.
Characteristics of a crystal lattice
- Each point in a crystal lattice is called lattice point or lattice site.
- Each point in a crystal lattice represents one constituent particle which may be an atom,a molecule,or an ion.
Types of crystal system
- PRIMITIVE UNIT CELLS.
- CENTERED UNIT CELLS.(These are of 3 Types.)
1- PRIMITIVE UNIT CELLS:- These are unit cells which have points only at the corners.They are also called simple unit cell.
2- BODY CENTERED UNIT CELLS:- The points are present at the corners as well as at the body centre of unit cell.
3- END CENTRED UNIT CELLS:- The point are present at all the corners and at the centre of any two opposite faces.
SEVEN CRYSTAL SYSYSTEM
1- Cubic.- All the three axes are equal of length and are at right angles to each other (a=b=c,all angles=90o)
2- Tetragonal.-The three axes are at right angles to each other but only the two axes are equal (a=b not =c all angles=90o)
3- Orthorhombic.- It has three unequal axes which are at right angles to each other (a not=b not=c.all angles=90o)
4- Monoclinic.- The three axes are of equal length length which are inclined at the same angle but angle is not equal to 90o.
5- Hexagonal.- It has two edges of equal length (a=b) abd two angles of 90o and one angle of 120o.
6- Rhombbohedral.- The three axes are of equal length which are inclined at the same angle but the angle is not equal to 90o.
7-Triclinic.The three axes are of equal length,and all angles are diffrent but none is perpendicular to any of theothers.
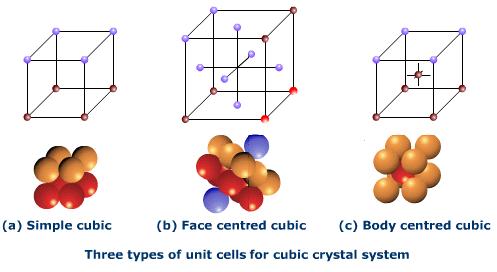
Three types of Cubic lattice
The three common types of cubic systems:
1- Simple or primitive. It has points at all the corners of the unit cell.
2- Body centered cubic unit cell (bcc). It has points at all the cornersas well as at the centre of the cube.
3-Face centered cubic unit cell (fcc)
TYPES OF CUBIC CRYSTALS AND NUMBER OF ATOMS PER UNIT CELL
The unit cell may be represented in three diffrent ways.
Simple or primitive cubic unit cell
Body centered cubic unit cell
Face centered cubic or cubic close packing unit cell.
(1)-- Simple or primitive cubic unit cell.
The number of atoms present in each unit cell = 8 corners X 1/8 atom per unit cell = 1 atom
(2)-- Body centred cubic unit cell.
The number of atoms present at the corners per unit cell = 8 corner atoms X 1/8 atom per unit cell= 1
Total number of atoms in bcc arrangement=1+1=2.
(3)-- The number of atoms present at corners unit cell = 8 corners atoms X 1/8 atoms per unit cell = 1
The number of atoms present at face unit cell = 6 atoms at the face X 1/2 atom per unit cell = 3
Total number of atoms in ccp or fcc arrangement = 1 + 3 = 4
STRUCTURES
CLOSED PACKED STRUCTURES
(A)-Close packing in one dimension
Coordination number : -The number of spheres which are touching a given sphere is called its co-ordination number.
(B)-Close packing in two dimensions
(C)-Close packing in Three dimensions
INTERSTITIAL SITES OR INTERSTITIAL VOIDS
1- TETRAHEDRAL SITE OR VOID.-The vaccant space amoung four spheres having tetrahedral arrangement is called tetrahedral site or tetrahedral void.
2-Octahedral site or void.-The void formed by two equilateral triangles with apice opposite direction is called octahedral site or octahedra void.
IMPERFECTIONS IN SOLIDS =
An Ionic crystal which has the same unit cell containing the same lattice points throughout the whole of crystal is known asideal crystal
Types of imperfections:-
1.Electronic imperfections 2.Atomic imperfections or point defects.
1-Electronic imperfections:- These correspond to defect in ionic crystals due to the electrons.
2-Point defects or Atomic Imperfections:-The defects which arise due to the irregularities or deviations from ideal arrangement of atoms around a point or an atom in a crystslline substance are called point defects or atomic imperfections.
The point defect arises due to any one of the following cases
(i) Vaccancy defect. When some of the lattice sites are vacant,the crystal is said to have vacancy defect.
(ii) Interstitial defect. When some constituent particles occupy vacant interstital positions,the crystal is said to have interstitial defect.
A. Defects in stoichiometric crystals.
B. Defects in non-stoichiometric crystals.
C. Impurity defects
A.Point defects in stoichiometric crystals Stoichiometric compounds. Are those in which the number of positive and negative ions are exactly in the ratios indicated by their chemical formulae.In these types of compounds two types of defects are generally observed.
1.Schottky defect
2. Frenkel defect
1.Schottky defect This defect was discovered by German scientist Schottky in 1930. It arises if some of the atoms or ions are missing from their normal lattice sites.The lattice sites which are unoccupied are called lattice vaccancies or holes. Conditions causing Schottky defects.This type of defect is usually observed in strongly ionic compounds having (i) High co-ordination number,and (ii) Ions of almost similar sizes. Eg-Nacl,KCL,Cscl.
This defect is also known as interstitial defect.
Conditions causing Frenkel defects.
- Co-ordination number is low.
- Anions are much bigger in size than the cations.
B.Point defcts in Non-stoichiometric crystals The compounds in which the ratio of positive and negative ions prsent in the compound differ from that required by ideal chemical formula of the compound are called non-stoichiometric compounds. Two types of point defectsmetal acess and metal deficiency defect. (A) Metal Excess defect-May arise due to the following two ways.
- Presence of cations in interstitial sites.
Consequences of Metal Excess Defects
- The crystal with metal excess defects are generally coloured.
(B) Metal Deficient Defects-These may arise due to two ways.
- Extra anions occupying interstitial sites
(C) Impurity Defects
These defects in ionic crystals arise due to the presence of some impuritiy ions at the lattice sites.
PROPERTIES OF SOLIDS
There is a close releationship between the properties of a solid and its composition and structure.some of them are :-
(1.) Electrical Properties of solids
Solids exhibit an intresting range of variation of electrical conductivities extending over 27 orders of magnitude.
Solids can be classified into three types:(i) Conductors (ii) Semi-conductors (iii) Insulators In metals,conductivity strongly depends on the number of valnce electrons available per atom.
(2) Magnetic properties of solids:- Each electron in atom has magnetic moment which originates from two sources:
(i) Orbital motion around the nucleus.
(ii) Spin of electron around its own axis.
External link's
1-en.wikipedia.org/wiki/Solid-state_chemistry
2-en.wikipedia.org/wiki/Cubic_crystal_system
3-en.wikipedia.org/wiki/Crystal_structure
4-www.science.uwaterloo.ca/~cchieh/cact/c123/bcc.html
5-chemed.chem.purdue.edu/genchem/topicreview/bp/ch13/unitcell.php
