User:Phaello/sandbox/Chemistry/Ionization Energy
Contents
Ionization Energy
This is the energy needed to remove the most loosely held electron in atoms. It should be in atoms that make up one mole. One mole is a measure of a certain number of atoms. Ionization energy is measured in Kilo Joules per mole (KJ mol-1 ). The Ionization energies for elements in the periodic table vary from around 380 KJ mol-1 to about 2370 KJ mol-1. Those elements that have high ionization energy do not normally form positive ions because of the large amount of energy they would need in order to loose an electron.
Ionization energy is needed to remove an electron from an atom. Remember: it is protons (nuclear charge) in the nucleus that hold the electrons tight to the atom. Ionization energy has to overcome the attraction the electron is having from the nucleus.
If there is a high attraction between the electron and the nucleus, the energy needed to remove the electron will be high.
As you move from left to right across the period the number of protons increases, thus increasing the effective nuclear charge. Take a moment and think, what will happen to the ionization energy as you move from left to right across the period in the periodic table? Yes, the ionization energy will increase.
Hydrogen has one electron in its outer most shell, which is shell number 1, closest to nucleus. Therefore the electron is strongly attracted to the nucleus. Hydrogen has no electrons shielding/screening the electron from the nucleus, so ionization energy is high – 1,310 KJ mol-1.
Helium has 2 electrons in the first shell – its ionization energy is even higher, 2,372 KJ mol-1 – nuclear charge is +2.
Periodic Table showing First Ionization trends of elements
What happens with effective nuclear charge as you move down a group in the periodic table?
As you move down the group the effective nuclear charge increases. How does the attraction between electrons in the outermost shell and the nucleus change? Does it become weaker or stronger? What are the reasons for that?
Because of the increasing distance, as the number of shells increases between the nucleus and the outermost shell, the attraction becomes weaker.
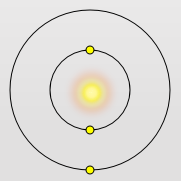
Lithium Atom
Lithium has the outermost electron further away from the nucleus The outer most electron is screened by 2 electrons – so does not get a full pull of the nucleus – its ionization energy is 519 KJ mol-1
Ionization energies in period 2 and 3 follow the same pattern It is just that ionization energies in period 3 are lower than ionization energies in period 2
So what will happen to the ionization energy as you move down the group?
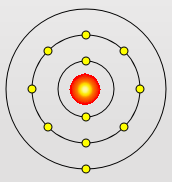
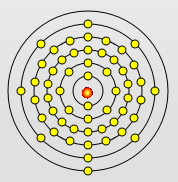
Sodium Atom
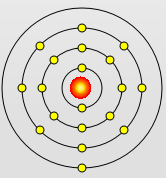
Potassium Atom
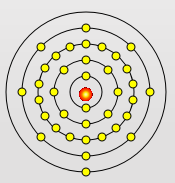
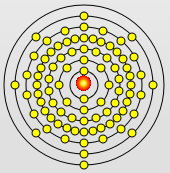
Rubidium Atom
Cesium Atom
Francium
As you move down the group the number of electrons shielding the outermost shell from the nucleus increases. That make the attraction of electrons in the outermost shell by the nucleus weaker.
The ionization energy will decrease because of the decrease in the attraction of the outermost electrons by the nucleus.