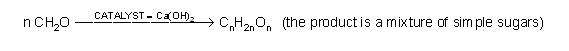Chemistry/Enriching Chemistry Teaching
Contents
- 1 Introduction
- 2 The “Poof” Rule
- 3 Some General Tidbits (To toss into a lesson that otherwise might be a bit boring)
- 4 CHEMWORDS
- 5 Useless Facts for the Day
- 6 Chemistry 11 Tidbits
- 6.1 Unit I : Safety in the Chemical Laboratory
- 6.2 Unit II: Introduction to Chemistry
- 6.3 Unit III: The Physical properties and Physical Changes of Substances
- 6.4 Unit IV: Inorganic Nomenclature
- 6.5 Unit V: The Mole Concept
- 6.6 Unit VI: Chemical Reactions
- 6.7 Unit VII: Calculations Involving Chemical Reactions (Stoichiometry)
- 6.8 Unit VIII: Atoms and the Periodic Table
- 6.9 Unit IX: Solution Chemistry
- 6.10 Unit X: Organic Chemistry
- 7 Chemistry 12 Tidbits
- 8 Unit II: Equilibrium
- 9 Unit IV: Acids, Bases and Salts
Introduction
Teaching is more than just delivery of knowledge, as every teacher knows. I found that if I could accompany my lessons with “tidbits” of information that students found interesting, the students enjoyed their lessons to a much greater degree. They looked forward to learning the extra bits that enlivened their time in class and, in addition, the lessons seemed to “stick in their heads better”. Above all, don’t forget the positive effect of humour on memory. The teacher doesn’t need to be a stand up comedian (which in fact is distracting) but a little humour can lighten the tone just enough to be effective. When students told me how much they enjoyed coming to Chemistry, I knew that the minute or two of class time needed to enliven the lessons were being paid back very handsomely.
The material that follows consists of anecdotes, analogies, “useless facts”, bits of chemical history, chemical humor and applications of chemical knowledge. The material was accumulated over a 40 year period and, it is hoped, will help other teachers to enliven their lessons and make students want to run to get to class. How can you assemble your own stockpile of “tidbits” to augment the material below? Read!! Read science-related material voraciously and let students know about the great stuff you find! Your enthusiasm for science should be enthusiastic, broad-ranging and infectious. Don’t just read stuff on chemistry; read up on biology (evolution theory, molecular biology, animal traits, etc.), physics, cosmology and astronomy, new technologies, modern mathematics – they are all good stuff. Some great sources are Science News, Chem 13 News, the Journal of Chemical Education and New Scientist (this last one is pricey but maybe you can convince your library to order it for the science teachers as an ongoing inservice item – divide the price by the number of science teachers in your school to make it sound cheap like borsch!). In addition, science books for the layman almost always spice up the text with neat and interesting tidbits. If you are spectacularly lucky, you might find old copies of Chem 13 News and the Journal of Chemical Education. Some university libraries may have copies, or even a complete set. Look for them and be prepared to photocopy the good stuff. Two weeks before I first started teaching, I went to the UBC library and spent $50 photocopying some wonderful content that served me well over the years.
In addition to the material presented below, one of the best ways to enrich the teaching of chemistry is to do demonstrations and more demonstrations. I found that keeping a special drawer for demonstration equipment and a special shelf stocked with solutions needed for demonstrations allowed me to quickly grab some chemicals and special pieces of glassware, tubing or whatever and assemble a demo at a moment’s notice. Your students’ enthusiasm for demos will amply repay the time it takes to prepare and perform them.
A caveat: The information presented below is what I remember and may not be 100% accurate in all cases. My memory is quite good for tidbits but memory can deceive, as the inaccuracies of eye-witness testimony in court have shown. Where I could find an original reference, I have quoted it. Otherwise, my memory or a scrap of paper with no way to identify the source has had to serve.
The “Poof” Rule
Many students are hampered by less-than-stellar math abilities. They have an incomplete understanding of what to do and when to do it when required to solve relatively simple algebra equations. Chem 11 does not require much algebra but solving equilibrium problems (Unit II in Chem 12) gives many students a severe case of math anxiety. For example, a simple problem such as solving ![]() for X creates difficulties for a substantial number of students. Rather than complaining about the situation and resigning yourself to demonstrations of poor equation solving performance, use the “Poof” Rule to bring a novel and logical approach to solving simple algebra problems that grabs kid’s attention and makes them capable of carrying out the required algebraic solutions.
for X creates difficulties for a substantial number of students. Rather than complaining about the situation and resigning yourself to demonstrations of poor equation solving performance, use the “Poof” Rule to bring a novel and logical approach to solving simple algebra problems that grabs kid’s attention and makes them capable of carrying out the required algebraic solutions.
Note: there is nothing fundamentally new about the “Poof” Rule but it brings a sufficiently-different viewpoint to students to allow them to clarify what is otherwise a muddied and jumbled perception.
Let’s use a specific example: solve the following for X. (This is big enough to grab their attention and help them focus on what you are going to tell them.)
Most students take several lines to arrive at the answer and most still get the wrong answer. Tell students you are going to show them how Math teachers do such a problem in their head and caution them not to reveal this secret method to others. In particular, Math teachers might get upset if they knew their secret mental method was known to lesser mortals. (Of course, students are encouraged to use the “Poof” Rule to check their results obtained by the usual math methods.)
First point out that two different situations arise when working with algebraic equations.
- Situation 1: Isolating the variable
- Situation 2: Applying mathematical operations to calculate an answer once a variable has been isolated
Students previously have been made to memorize the correct order in which to apply mathematical operations when manipulating numbers (Situation 2) - BEDMAS (order of operations is: “Brackets” first, then “Exponentials”, then “Division” and “Multiplication” and finally “Addition” and “Subtraction”). BEDMAS applies when you have to evaluate the result of a series of operations. For example, evaluate X in :
They know (or should know) that they must first evaluate the terms inside the parentheses, square the result inside the parentheses, subtract 1, and finally divide by 4 to get an answer of 2.
What students are not so clear about is the fact that when isolating the variable, the operations are used in REVERSE ORDER: SAMDEB. Since solving for X consists of stripping off the operations which were grouped around an X, the operations must be removed in the opposite of the order that would be used to simply evaluate an expression. Now, Math teachers may insist that students solve a simple equation in the following manner:
But in fact Math teachers actually do the solving in their head this way: “Since I want to get X by itself and since there is a ‘+2’ which is on the X side, I will simply move the ‘+2’ to the other side in such a way that it disappears from the left side and ‘Poof’ it appears on the other side as the opposite of an addition; that is, it appears on the other side as ‘-2’.”
Hence, the “Poof Rule” is: “Strip off the terms associated with X (in the order SAMDEB) in such a way that the terms disappear from the side containing X and “Poof”, appear on the other side as the OPPOSITE operation”. (“Addition” and “subtraction” are opposites of each other, “multiplication” and “division” are opposites of each other and “taking the square” and “taking the square root” are opposites of each other.) The monster equation above can be solved in what is essentially one line by using parentheses liberally whenever operations are grouped, or by using a calculator and pressing the “=” sign after each addition, subtraction, multiplication and division operation. (An “=” sign is automatically appended when the square and square root operations are used on a calculator.) When demonstrating the idea on a blackboard, erase an operation from the “X” side, say “poof” and write down the opposite operation on the other side, slowly stripping the “X” side down to a bare “X” and building up the other side as a series of numbers and operations grouped with parentheses. The above monster equation, can be solved in one line using the “Poof Rule”, but the first few “Poof” operations are shown for purposes of clarity.
The original equation:[math]\frac{\sqrt{\frac{2 \left(3X^2-5 \right)-2} {6}}+6} {4}=8[/math]
“Poof”, becomes [math]\sqrt{\frac{2 \left(3X^2-5 \right)-2} {6}}+6=8*4[/math] (the division sign isn’t needed if there is just a “1” below)
and “Poof” becomes [math]\sqrt{\frac{2 \left(3X^2-5 \right)-2} {6}}=\left(8*4\right)-6[/math]
and “Poof” becomes [math]\frac{2 \left(3X^2-5 \right)-2} {6}=\left(\left(8*4\right)-6\right)^2[/math]
and so on, until [math]X=\frac{\sqrt{\frac{{\left(\left(8*4\right)-6\right)^2}*6}{2}+5}}{3}=26.07041746...[/math]
(On a calculator, one would press the key sequence: 8, X, 4, =, –, 6, =, X2, +, 2, =, X, 6, =, , 2, =, +, 5, =, , 3, =,[math]\sqrt{X}[/math] . The answer should appear as 26.07041746)
One other useful tool that not all students seem to use as often as they should is the idea that if the unknown is in the denominator of a fraction the denominator involving X should be “swapped” in a kitty-corner fashion with the numerator on the opposite side of the equation, as soon as possible. For example, in
[math]\frac{9}{\left(X-3\right)^2}=6[/math], swap the [math]\left(X-3\right)^2[/math] and the 6 to produce [math]\frac{9}{6}= \left(X-3\right)^2[/math]
Some General Tidbits (To toss into a lesson that otherwise might be a bit boring)
When a saturated solution of calcium chloride (available commercially as driveway de-icer) is added to ice chips, the temperature drops to -40oC. (Note that instead of melting the ice, the calcium chloride makes the ice even colder. Teacher: this is a result of freezing point depression due to the decrease in vapour pressure of the calcium chloride, which causes the vapour pressure of the ice to be lowered by decreasing the temperature. This is actually a first-year college chemistry topic so just treat this as a weird fact to be explained another year.)
Occasionally, students light a Bunsen burner and find that the flame burns a few centimeters above the top of the burner. Many will just accept that this is just some weird thing that Bunsen burners do but a few will actually wonder WHY this behavior occurs. The reason for this behavior is that when a gas expands through a small hole, the gas cools (the energy needed to overcome the intramolecular attractive forces is taken from the kinetic energy of the molecules.). As a result, it takes a few centimeters of travel before the gas molecules warm up enough to burn. Students find this “hovering flame” effect is most noticeable when the gas valve is turned up too high. The cooling effect, known as Joule-Thompson cooling, also is responsible for the formation of dry ice when the gas in a tank of compressed carbon dioxide is allowed to expand through a tiny orifice. (The opposite effect is the heating of a gas when it is compressed, which is readily observable when a tire pump is used to inflate a tire and afterwards the barrel is felt to be warm.) [Chem 13 News, Feb. 2001, p. 4]
The average age of people suffering from brain aneurysms is 50-60. However, a study showed that the average age of brain aneurysms in cocaine users is 37. In the study, problems were found to occur within 6 to 12 hours of taking cocaine (which dilates blood vessels as a result of increased blood pressure, and which causes a previously weak blood vessel to “blow out”). One third of the cocaine users affected were unable to move their arms or legs, some could not speak or even understand speech, and others had a facial droop to one side. Almost half of those affected suffered a stroke. (Aneurysms occur when a pre-existing dilation of a blood vessel is aggravated, causing the blood vessel to burst.) [New Scientist, November 21, 1992, p.16]
A fluorescent chemical is a chemical that gives off light and glows when exposed to light. Many detergents contain fluorescent dyes that glow when exposed to sunlight. The idea is to convince you that the clothes are “so clean they glow”, regardless of how clean (or dirty) they really are. The fluorescent dyes do not make the clothes cleaner and in fact do nothing but make the clothes glow. Likewise, some eye drops contain fluorescent dyes that glow when exposed to sunlight, to convince you that the eye drops have made your eyes so healthy they glow.
Several years ago, a toothpaste advertised itself as “the toothpaste with sex appeal”. Their secret ingredient was … chloroform! The chloroform acted as an anaesthetic and made the tongue numb for a second; when feeling returned, the tongue had a “pins and needles” feeling. Apparently someone in an advertising agency believed a numb tongue was “sexy”. (It is fortunate that the chloroform was eventually removed for the toothpaste because chloroform is now known to be a carcinogen; that is, a chemical which increases the chances of getting cancer.) While on the topic of toothpaste, several toothpaste manufacturers in the 1950’s included chlorophyll in their products. Chlorophyll is the chemical that gives plants their green colour and which is used by the plant to convert sunlight into chemical energy. Presumably, the idea was that if chlorophyll is good for plants, it is good for humans. Unfortunately, humans don’t use photosynthesis as a direct energy source.
Most detergents contain specific chemicals that create lots of bubbles. In fact, the bubbles have no effect on the cleaning action but people have been conditioned to believe that the longer the bubbles last when using a detergent, the better the detergent must be. The user is supposed to believe that as long as bubbles are present, the detergent still has cleaning action. Unfortunately, the foaming agents have no effect on the cleaning ability of a detergent: the agents simply produce suds. By adding a foaming agent to plain tap water and then shaking the mixture, you would end up with bubbles overflowing the container. Several years ago, a firm in Richmond B.C. produced a truly superior detergent which most users claimed was far better than anything they had ever tried before. However, all the foaming agents normally used in detergents interfered with the cleaning action of this remarkable detergent. The company went broke because they could not find a foaming agent that produced enough bubbles when added to their detergent. Not enough consumers would use a detergent that didn’t produce bubbles, in spite of the fact that the bubbles served no purpose.
Cyanide is usually thought of as an extremely poisonous substance, which of course it is. The following minimum lethal doses (in moles per kilogram of body mass) show how much more poisonous some other chemicals are.
Sodium cyanide (2.0 x 10-4)
Strychnine (1.5 x 10-6)
Bufofotoxin [from a frog that is poisonous to the touch] (5.2 x 10-7)
Tetanus toxin (1.0 x 10-15)
Botulinum toxin A (3.3 x 10-17; How many molecules are in a lethal dose here?)
[Science News, September 3, 1983, p.157]
The Student’s Guide to Problem Solving:
- if at all possible, avoid reading the problem. Reading the problem only consumes time and causes confusion.
- Extract the numbers from the problem in the order in which they appear. Be on the watch for numbers written in words.
- If rule 2 yields 3 or more numbers, the best bet for getting the answer is to add them together.
- If there are only two numbers that are approximately the same size, then subtraction should give the best results.
- If there are only two numbers in the problem and one is much smaller than the other, then divide if it goes exactly, otherwise multiply.
- If the problem looks like it calls for a formula, pick a formula that has enough letters to use all the numbers in the problem.
- If rules 1-5 don’t seem to work, make one last desperate attempt. Take the set of numbers found by rule 2 and perform about 2 pages of random operations using these numbers. You should circle about 5 or 6 answers on each page just in case one of them happens to be the answer. You might get some partial credit for trying hard.
- Never, never spend too much time solving problems. This set of rules will get you through even the longest assignments in no more than 10 minutes with very little thinking.
[Joe Dodson, Mathematics Supervisor, Winston-Salem/Forsyth County Schools, North Carolina; quoted at http://dbhs.wvusd.k12.ca.us/Humor/Student-Prob-Sol-Guide.html]
Actual excerpts from student science exam papers:
- To remove air from a flask, fill it with water, tip the water out, and put the cork in quick before the air can get back in.
- The process of turning steam back into water again is called conversation.
- A supersaturated solution is one that holds more than it can hold.
- When you smell an odourless gas it is probably carbon monoxide.
http://dbhs.wvusd.k12.ca.us/Humor/Student-Mistakes.html
Logical Names for Element Symbols
Although there are many attempts to associate the symbols of element with the names of everyday things (for example, CrO2-4 = a black bird’s wife, Si = a goofy prisoner, etc.) the following symbols of elements are given more appropriate, humorous, names.
- Rn = Nursium
- Dy = Princessium
- Pa = Daddyum
- Ra = Cheerium
- Br = Freezium
- Cu = Farewellium
- No = Negativium
- Pu = Stinkium
- Y = Questionium
- Bi = Solongium
- Li = Notruthium
- F = Mygradeinchemistryium
[Chem 13 News January 1989, p.5]
Favorite Elements of Various Characters
| Who? | Element |
|---|---|
| Eskimo | “Burrr”-ylium |
| Undertaker | “Bury’em” |
| Physician | “Heal”-i’em |
| Cowboy | “You Rope-i-‘em” or “Rode”-ium |
| Escaping convicts | Are-gone |
[J.Chem.Ed. Vol.76, No.4, p.493]
Chemical Sniglets
[The following made-up words were published in Chem 13 News, February&March 1987, p.12.]
Off der Waals forces = forces that drive a chemistry student crazy (when studying Chem 11, Unit VIII)
Chemfusion = the turmoil created when a teacher announces a test on chemical bonding.
Chemache = any fabricated ailment used as an excuse to get out of chemistry class.
Students Say the Darnedest Things
(The student bloopers below were collected in the year following the blooper. Student confusion is not a new phenomenon!)
A formula is a group of letters that can be broken up into elements. (1925)
A gas is a dry liquid. (1925)
Gunpowder is mixed thoroughly so as to fool the ingredients into thinking they are a compound. (1926)
Temporary and permanent hardness of water signifies whether the water is in the form of snow or ice. (1926)
To tell which of two samples of ammonia water is the stronger, smell each of them, and the one which makes the most tears come in your eyes is the stronger. (1926)
We breathe out carbon dioxide and beverages breathe it in. Beverages need carbon dioxide to grow. (1926)
Water exists in two states, tap water and distilled water. (1928)
[J.Chem.Ed. Vol.76 No.7, p.888]
CHEMWORDS
Challenge your students to come up with words and names in various categories, using only the symbols of chemical elements. Some examples:
- Capital cities: LiSbON, PArIS, NaIrOBi, CaIrO, CaNBErRa
- Long words: ClAsSiFICATiON, PrOCrAsTiNaTeS, InFeCTiOUSnEsS
- Names: AgNeS, FRaNK, ClArK, LaUReN
[Chem 13 News, March 1994, p.1 and May 1994, p.3
Useless Facts for the Day
|
I tried to introduce each lesson with a “useless fact for the day”. Sometimes the fact was a way to introduce a topic and sometimes it was just a neat thing that I had read. Either way, students soon ran to class (because the fact was delivered in the first minutes of class) and would even complain when I didn’t have a useless fact for the day. Here are a few “useless facts for the day” – use them at your own risk. |
Spider webs usually have stiff radial strands and elastic strands in the “capture spiral”. Biologists found that the capture spiral strands of the webs of “orbweb” spiders have tiny “globs” of liquid spaced along the strands. When examined more closely, the globs were found to consist of a thick liquid glue encapsulating a randomly-coiled part of the strand. When an insect hits the web, the capture strands might snap if it were not for the coiled bits of strand inside the loops acting as a bungee cord. The impact of the insect on the radial strand uncoils some of the coiled material inside the viscous glob and absorbs the impact like a bungee cord. [New Scientist, August 19 1989, p.29]
Some biologists found that it took about 7 trials before they could train an earthworm to consistently turn either to the left or right of a simple T-maze (go the correct way and get some nice juicy mud or go the wrong way and get a mild shock). Earthworms aren’t too bright. Then, other scientists found that if a human baby was confronted by a flame behind a piece of glass, it took about 7 repetitions to train the child NOT to try to touch the flame. Humans apparently aren’t fast learners either. Reason: humans and earthworms share similar nervous systems with similar characteristics; humans of course have much more complicated systems but the basic components are almost identical.
Some scientists wondered if the effects of various illegal drugs such as cocaine had an effect of nervous systems other than human systems. To investigate, they gave samples of cocaine to spiders and found that spiders that previously produced beautiful and symmetric webs now produced webs with strands that were a hopeless jumble of threads. Other drugs such as caffeine, nicotine, marijuana, heroin, crystal meth and a few others similarly addled the spider’s abilities to function in a normal manner.
Humans are not the only species that will swear. Koko, a gorilla, was trained in sign language to understand many human words and commands. Koko quickly picked up on the fact that humans do not like dealing with excrement. When Koko got frustrated, as often occurred, he would spontaneously make up new phrases, using sign language, such as “(Trainer’s name) is dirty (term for excrement). Koko is nice”.
Scientists have found that phenylethylamine is a chemical produced in the brains of people who fall in love. (Once people cease being in love their brain stops producing the chemical.) Interestingly, the same chemical is found in chocolates, which may be why people who have been jilted often eat large amounts of chocolate, perhaps unconsciously trying to duplicate the heady feeling of romance.
A chemist may have inadvertently killed Napoleon. When Napoleon was captured after his defeat at Waterloo, he was exiled to St. Helena, which is a cold, damp island in the Atlantic. Napoleon shared a residence with the British commander of the island and was given free run of the island (where would he go?). At that time, in 1812, a very fashionable type of wallpaper used a beautiful green pigment invented by a Swedish chemist: Scheele’s green – copper(II) hydrogen orthoarsenite, possible formula = CuHAsO3. When mold forms on the green pigment, some of the chemical is converted to arsenic trimethyl, which is a very toxic gas. The French have long accused Britain of secretly poisoning Napoleon to prevent him from escaping again. Analysis of samples of his hair showed harmful amounts of arsenic present, but in fact Napoleon may well have been poisoned by the wallpaper in his host’s house. Napoleon’s doctor recorded the symptoms of his illness, and those symptoms agree with arsenic poisoning. Interestingly, his companions also had the same symptoms, which would be expected if they breathed the same poisonous gas. Chemistry again helped shape the course of world events! [New Scientist, October 14, 1982, p.101]
A chemist has produced strong evidence that Napoleon’s march into Russia may have been doomed by a phase change in tin metal. Napoleon’s army was probably the best-outfitted in Europe at the height of his success. Among other things, his soldiers had coats and trousers with shiny tin buttons, which kept their silvery shine for a long time and were considered far better than the common brass buttons of lesser soldiers. The modern day chemist pointed out that at low temperatures, such as was found in the Russian winter of 1812, shiny, hard tin changes phase from the shiny white beta form to the grey and powdery alpha form. Hence, as winter progressed, the soldier’s buttons would have disintegrated and they would have found themselves with coats that couldn’t be button closed and trousers that couldn’t stay closed or stay up. The final result was a soldier that was getting very cold and was probably hampered in trying to fire a weapon. Chemistry may have stopped Napoleon’s attempt to conquer Russia. [New Scientist, September 11, 1986, p.57]
The ancient practice of bloodletting was not as barbaric and senseless as modern physicians previously thought. Research has shown that if a person has a dangerously high fever and a substantial amount of blood is drawn from the body, the brain detects the drop in blood pressure and releases a neurotransmitter called argenine vasopressin, which depresses the body temperature and “breaks” the fever. The effect, although not the reason why, was well known to the early Greeks. [Calgary Herald, August 9, 1992, p. B7]
It is well known that the science of genetics was started by the Austrian monk Gregor Mendel, who experimented with pea plants (although his results were ignored and forgotten for many years.) What is less well known is that he fudged his results. The pea plants he worked with were very prone to mutations, “spoiling” his results. Since he thought natural laws must be perfect as a logical extension of divine laws, he assumed he had made errors and fudged his results in order to make his results perfect. (Perfect results are impossible with such pea plants, as shown in experiments trying to duplicate Mendel’s results.)
Viewers of the movie “Titanic” may remember that Leonardo DiCaprio clung to a floating raft for what seemed to have been hours, talking to the heroine, before finally freezing and slipping into the deep. That scene must have been filmed in slow motion because a person in ocean water cooled by ice has no more than a minute or two, at most, before losing consciousness from the cold. Kayak races in glacier-fed streams used to require rescue people suspended above the stream every hundred meters or so because within about 30 seconds of immersion an unprotected person falling into such cold water is incapable of using their arms to swim.
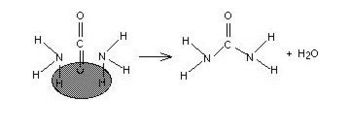
Alligator hunters are able to find areas where the reptiles live by a simple method: they detect the smell of ammonia in alligator urine. One of the by-products of protein digestion is ammonia, which alligators eliminate directly via their urine. Humans and other mammals combine the ammonia (which is quite caustic) with carbon dioxide to produce urea (NH2CONH2) and eliminate it as shown in the drawing below.
Most coal deposits contain a few parts per million of uranium. If the uranium present in coal is separated out and used in a nuclear reactor to produce energy, a quick calculation shows that there is more energy content in the uranium present in the coal than can be obtained by burning the coal to make electricity. In other words, the best way to use coal might be to separate out the uranium, throw out the coal and just use the uranium for energy production.
Some chemical companies sell vials containing tiny amounts of a very expensive compound called “cadaverine”. The compound has the distinctive odour of decomposing human corpses and vials of the compound are used to train dogs to find buried bodies.
Some strain gauges are now so sensitive that they could detect the bending of an aircraft carrier deck (more than 6 inch thickness of strong steel) when a fly lands on the deck.
Japanese researchers have now found a way to make diamond from vodka. They vaporize a water-alcohol mixture (vodka) and subject the vapour to intense microwave energy, causing a deposit of pure polycrystalline diamond to coat the inside of the microwave cavity. By placing different objects inside the microwave cavity, they are able to diamond-coat different metals, including speakers cones, tool bits, and so on.
One of the most remarkable cases of suspended animation occurs with Texas cattle ticks. For years they sit on a bush with no visible signs of life. Nevertheless, if a cow passes by the bush, the tick immediately comes to life and jumps onto the cow as it passes by the bush.
It is well known that bats use pulses of ultrasound to find objects such as flying moths in the dark, in the same way that a radar system observes flying aircraft. What is less well known is that moths have adapted to this threat from bats by being covered in a radar-absorbing fur that lessens the radar signal bouncing back to the bats ears. [“In the Blink of an Eye”, Andrew Parker, Basic Books, New York, 2004. p.94]
Geologists frequently talk about a layer of iridium all over the earth that was laid down 65 million years ago when a large asteroid, estimated at 10 km across, impacted the Gulf of Mexico and precipitated an extinction event, ending the dominance of the dinosaurs. An interesting accident occurred when a technician was measuring the iridium concentration in a sample of clay at the Cretaceous boundary. Walter Alvarez, who headed the study noted that one measurement was ridiculously high “due to the platinum wedding or engagement ring worn by a technician who had prepared the samples. Platinum used for jewelry contains about 10 percent iridium… If a platinum ring loses 10 percent of its mass in 30 years, the average loss per minute, if it all deposits on a sample, is about one hundred times higher than our sensitivity of measurement.” Hence, Alvarez concluded that a few seconds exposure to a platinum ring is enough to produce a completely spurious iridium signal. The average iridium concentration of crustal rock is less than one tenth of a part per billion, while genuine iridium “signals” from the Cretacous boundary is about 6 parts per billion. [“Comet”, Carl Sagan and Ann Druyan, Random House, New York, 1985, p.282]
Carbohydrates got their name from the fact that an early chemical analysis of sugars showed that they had the formula CNH2NON. Since the existence of hydrates was known, it was assumed that sugars were simply hydrates of carbon, so that sugars were CN•NH2O. One French chemist spent virtually his entire career in a futile search for ways to form carbon hydrates by adding water to carbon using various catalysts, temperatures and pressures. Unfortunately, what was not known at the time was the carbons in carbohydrates exist in a long chain such that most carbons had an “H” and an “OH” attached: H-C-OH.
An ingenious scam relied on the inability of most people to understand probabilities. The scammer sent out an investment newsletter and claimed to be an extremely wealthy investment advisor who was now semi-retired and just wanted to help others make millions like he did. Pretend you received the first newsletter, free of charge, and the “investment advisor” discussed a certain stock and predicted that it would gain in value within a week. You wait and sure enough the stock goes up. The next newsletter discusses another stock and advises to sell it because it will go down within a week. Within the week, the stock goes down, as predicted. The newsletter is sent to you every two weeks for 16 weeks and each time the prediction is dead on. Then, a letter comes from the investment advisor asking you to send him $1000 to continue getting the newsletter for the rest of the year since he is wealthy but not excessively rich and he needs to at least cover his expenses. He points out that if you started with a $1000 investment 16 weeks ago and had followed his advice you would have made over $5000 in that time. Since such newsletters usually cost between $10,000 to $25,000 per year, his request is a bargain. Question: would you send the $1000 to the investment advisor? [If you did, you will have wasted your money. The scammer starts by sending out 128,000 letters, 64000 of which said the 1st stock would go up and 64,000 said the stock would go down. He always picked “volatile” stocks which constantly went up or down so that “no change” was highly unlikely to occur. He then ceased sending the letter to those that predicted the wrong “movement” for the stock. The next week he sent 32,000 letters saying the next stock would go up and 32,000 saying it would go down. This continued for 8 newsletters until only 1000 newsletter recipients were left. Usually about 50% of the recipients who had witnessed this “miraculous” set of 8 correct predictions would send in $1000, for a nice take of $500,000. True, the scammer still had to keep sending the newsletter to those who sent in their $1000, but strangely his predictions were now no better than random hits.]
During the 1982 Falklands War between Great Britain and Argentina, Argentina launched an Exocet missile against the British destroyer HMS Sheffield. The missile came in low and struck the upper part of the ship called the “superstructure”. Initially the resulting fire was thought to be easily containable but as soon as seawater was poured on the fire it rapidly flared out of control. The reason for this sudden flare-up was the fact that the superstructure was made of an aluminum alloy, which, like all aluminum, burns fiercely when water is added to already burning aluminum.
When liquid helium is cooled to below 2.2 K, its viscosity drops to zero and helium becomes a superfluid, wherein the atoms can move without friction. Helium has several interesting properties. For example, it never forms a solid, no matter how low a temperature it reaches. Also, if the liquid helium is set in motion, it continues to move since there are no frictional forces. In addition, when helium forms a superfluid it is able to self-siphon out of any open container. Finally, zero Kelvin is usually defined as the temperature at which all motion stops because atoms have no kinetic energy. However, when helium is cooled to within a tiny fraction of a degree away from zero Kelvin, it still continues to move. [For the teacher: Helium is a quantum liquid and as such has to obey the Heisenberg Uncertainty Principle which states that a particle cannot have both a definite position and a definite momentum. If a quantum particle such a liquid helium were to stop moving completely, it would therefore have a definite position and would have a definite momentum since it would have zero kinetic energy (momentum = mass x velocity). As a result, helium atoms at extremely low energies will still possess a zero-point energy and this energy is sufficient to dislodge it from a crystal lattice, preventing helium from freezing.] [New Scientist, November 25, 1995, “Inside Science: The Big Chill”]
In spite of all the advertising to the contrary, the fancy 'fizz keepers' that inject air into half-empty pop bottles simply DON'T WORK. Neither do simple stoppers, although they are a much cheaper way to achieve the same result: flat pop. Why? Bottles and cans of soda pop (and other fizzy drinks that some adults consume) contain dissolved carbon dioxide gas. There is a scientific law called Henry's Law which tells us that the amount of a particular type of gas, say carbon dioxide, that can dissolve in a liquid depends on the pressure of that same gas in the airspace above the liquid. Other gases in the airspace, such as oxygen and nitrogen, have no effect: only carbon dioxide in the airspace affects the amount of dissolved carbon dioxide. In plain English, that means the greater the pressure of carbon dioxide gas over water, the more carbon dioxide that dissolves into the water. When you open a bottle of pop, the carbon dioxide escapes from the airspace above the pop. Since the pressure of carbon dioxide above the water is now very low, the gas dissolved in the pop starts to come back out of solution and we see bubbles forming. What does that have to do with fizz keepers? Well, if you use a pump to increase the air pressure in the space above the pop, you increase the pressure of the gases contained in air, namely oxygen and nitrogen. However, air contains only a tiny amount of carbon dioxide, even when pressurized, so there is nothing to stop the dissolved carbon dioxide from continuing to come out of the liquid and the pop eventually 'goes flat'. A suggestion: if you are tempted to spend a great deal of money on a fancy fizz keeper, why not buy a cheap cork instead (to keep out refrigerator odors) and spend the extra money on a neat science toy?
Although a few air fresheners contain chemicals that evaporate and bind onto nasty smelling odors in the air, almost all air fresheners work in one of three ways. Some work by temporarily numbing the nerve endings in your nose, so that you can't smell much of anything (let alone bad smells). Still others contain oils that float through the air and coat the inside of your nasal passage and prevent you from smelling any foul odors in the air. The last type works by producing a smell that simply overpowers other odors and prevents you from noticing the bad smell. That's it. In all cases, the air in your room still has all the nasty chemicals in it that give rise to the bad smell; you just can't smell them anymore.
Aluminum can be vaporized at a relatively low temperature in a vacuum and this vapour condenses on any object it touches, so that a thin layer of aluminum metal can form a shiny, heat- and light-reflective coating on mirrors, decorative paper and plastic toys. Aluminum-coated Mylar plastic has saved the lives of several people who were lost in freezing conditions. By wrapping themselves with the heat-reflective plastic sheet, they were able to survive temperatures that would have otherwise threatened their lives. The heat-reflective property of these same sheets has also saved the lives of several fire fighters who were trapped when a forest fire roared down on top of them, cutting off their escape. By staying in a depression in the ground, covered by the plastic, the fire fighters were protected when the heat wasn’t able to fully penetrate the blanket.
Your teeth glow yellow-green in ultraviolet light!
There are many so-called psychics who claim to be able to predict future earthquakes. Several of the “best” earthquake predictors were contacted and asked if they would take part in a test of their abilities. If they were successful, they stood to claim a one million dollar prize. All they had to do was to predict where an earthquake would occur, when it would occur (within one year from the time of the prediction) and the approximate severity. They would be compared to a computer program that would make random predictions concerning places, times and severities. All agreed to be tested and all were certain they would win the prize. One year after the predictions were made by the psychics and the computer, the results were tallied. The winner was the computer. No psychic scored as well as the computer’s completely random predictions. Another test of paranormal abilities involved an ability to “dowse” and locate underground water. A course of underground pipes was laid out and the field of pipes was then buried. The pipes went in random directions and were at different depths located in a large field. Valves were included to allow a particular pipe to be empty of water altogether, filled with running water or filled with non-running water. Again, some of the “best water dowsers” in the world were contacted and after agreeing to be tested (because they knew they were positive they could win a $10,000 prize), they were flown (at the testers expense) to the testing site. The results were abysmal: no dowser scored better than would be expected from a random result. Incidentally, James Randi (Randi the Great, a magician) has now put up the million dollar prize to be claimed by anyone that can demonstrate any paranormal abilities under controlled conditions. Several hundred psychics, dowsers, mind readers, communicators with the dead, etc. have tried to win the prize and not one has come even slightly close to being able to demonstrate the abilities they claimed. [The Skeptical Inquirer, Journal of the Committee for the Scientific Investigation of Claims of the Paranormal, Fall 1979, p.16 and p.7]
The most valuable water in the world is not super-pure water created in a laboratory; it is some rust-contaminated and stinky water that was found in North Africa many years after the end of World War II. The rusty water was part of a forgotten supply cache left behind in the desert when the German army retreated. What makes the water so valuable is the fact that it is the only water in the world that has not been contaminated by radioactive fallout from atmospheric testing of nuclear weapons and so is used to determine the natural “background” levels of radioactivity in water.
The growth of children is not continuous (it was previously thought that children grew about 0.5 mm in body length per day). A detailed study showed that children stay exactly the same length for between 2 days to slightly more than 60 days and then within 24 hours they grow between 0.5 to 1.8 cm (0.2” to 0.9”). This unexpected result partly accounts for the fact that teenagers suddenly become clumsy (“He just doesn’t seem to know where his feet are”) and find that their clothes are too short, seemingly overnight. [Science News, Vol.141, p. 102]
Angel fish in the clear surface waters of the Amazon river fight for territory by aligning their flattened silvery bodies such that the bright sunlight is mirrored into the eyes of rivals, bursting blood vessels in the eyes and stunning or killing the opponent. [“In the Blink of an Eye”, Andrew Parker, Basic Books, New York, 2004. p.84]
Chemistry 11 Tidbits
Unit I : Safety in the Chemical Laboratory
A student of mine was just finishing a late lab, taking off her safety goggles only after clean up was completed. When she put on her coat, an open flip-top squeeze bottle of 6 M hydrochloric acid was found to have rolled under her coat. When she snapped down the lid to cap the acid bottle, a tiny drop of acid squirted from the lip of the bottle directly into her eye. She did not panic but immediately did two things: she called out to alert me of what had happened and immediately ran to the eye-wash fountain, which happened to stand beside her. Unfortunately, nothing came out of the eyewash because the water pressure was almost zero. I rushed her into my prep area and laid her back over a sink where water could run over her eyes while I called the local hospital to alert them that we were coming. After rinsing her eyes for 10—15 minutes I took her to the emergency department of the local hospital (5 minutes away) and was immediately confronted by an admitting nurse wanting all the details of her health insurance (while I wanted to simply scream “treat her now, we will worry about payment later!”. After she was finally admitted, the emergency room physician – who turned out to be her father! – determined that we had done all that we could and the damage was going to be permanent but relatively minor (a small spot on her cornea which she has learned to deal with subsequently). Knowing that such a horribly improbable sequence of events did happen, I pointed out to students that they must try to anticipate the possibility of unfortunate things happening to them and always keep their personal safety in mind. Low probability does not mean impossible!
Don’t assume all liquids are water: (1) A worker in the chemical industry sat on a chair that had a few drops of liquid on it. The liquid was nicotine (a purified liquid compound extracted from tobacco) and absorbed onto his pants and skin, passing through his skin into his blood system within seconds. The worked was seriously ill within seconds because nicotine is very poisonous. (2) A student came into a chemistry lab at the start of the day and accidentally touched a droplet of liquid on a lab bench, receiving second degree burns. Apparently, another student had been using sodium hydroxide pellets the day before and left a pellet on the bench. Sodium hydroxide pellets can absorb enough water from the atmosphere to dissolve themselves overnight. (This also explains why sodium hydroxide pellets must be weighed quickly: if left on a balance for more than a few seconds the mass reading continually increase as the pellets absorb water from the air.)
Unit II: Introduction to Chemistry
On two separate occasions, students came up to me after the final exam and asked what the answer was to a certain high-mark calculation question. Each time, when I told them the answer they told me something like “Yes! I got it! Could you tell me how to do the question? I couldn’t figure it out.” When asked how they got the answer, they each replied “Oh, I just used a unit conversion.” I related these conversations to my students just after I announced that we were going to learn how to use unit conversions and commented that the method is so powerful it should almost be illegal! Students seem eager to learn a method that can help find an answer even if they might not know exactly how to solve a problem.
Students might be able to remember the difference between weight and mass better if they consider the case of an astronaut working to assemble a space station. Pretend he finds that the sun is suddenly being blocked by a large section of the station that is rapidly heading towards him as he is working on a wall of the station. The section might well be weightless but it has a huge mass and can potentially do him considerable damage. (Weight is the attraction of gravity for a mass whereas mass is a fundamental property of matter. Weight does not exist in the absence of a gravitational field while mass is the same regardless of whether of not a gravity field is present.)
Some wharves, especially in the Red Sea, are now being made with a special type of cement that has such a small density that it floats like a cork. The secret to the low-density cement is the use of “sand” grains that are actually small, hollow glass spheres. The light-weight grains create a cement that is as strong as regular cement.
Exercise 39 on page 26 of “Chemistry 11: A Workbook for Students” was published several years ago (with very slight changes to avoid committing plagiarism) as an example of a problem which U.S. chemistry students found very difficult to solve! Tell your students this fact and watch their pride grow as they solve this supposed “stinker”!
A visual analogy for the difference between accuracy and precision is made by throwing darts at a dart board. If all the darts cluster tightly together, but are very much to one side of the board, the dart thrower is precise (the throws are very reproducible). If the darts are evenly scattered all over the board such that there are as many darts above as below and as many to the right as to the left, the dart thrower is accurate, but not very precise (reproducible). If the darts are clustered tightly at the center, the dart thrower is both accurate and precise.
Even Chemistry professors sometimes forget about precision. During the early 1960’s a Chemistry professor at UBC went to Germany on sabbatical leave to do spectroscopy research. Finding that the spectroscope he was using kept getting dust on it that interfered with his results, he ordered a dust cover to be made for it: 1 m x 0.5 m x 2 m in size. He expected a quick response and a box made out of thin plywood, forgetting that the shop to which he sent the request was used to making high precision instruments. After a few weeks, the box arrived. It was made of polished brass sheets and was 1.00000 m x 0.50000 m x 2.00000 m in size. The prof said that he was too embarrassed to admit to the craftsman that such precision wasn’t needed.
Unit III: The Physical properties and Physical Changes of Substances
Science is frequently portrayed as the ultimate triumph of experiment and logic, but in fact new theories are frequently disregarded by established scientists who have a huge emotional and career investment in a previous theory. Max Planck, the father of quantum theory once commented: “A new scientific truth does not triumph by convincing its opponents and making them see the light, but rather because its opponents eventually die, and a new generation grows up that is familiar with it”. [J.Chem.Ed. Vol.71, No.5, p.394]
“That went over like a lead balloon” is no longer a valid statement. Knowing that pure lead metal is very malleable, some scientists (who obviously had too much time on their hands) rolled some lead into an extremely thin sheet and made a balloon out of the sheet. When filled with helium gas, the balloon actually floated … not very high off the ground, but it did float!
Villagers on a hilltop in Chile’s Atacama Desert (the driest desert in the world) are being supplied with 11,000 L per day of pure water from 75 sheets of plastic mesh (12 m x 4 m, 2 m off the ground) that condense the fog coming off the ocean on an almost daily basis. The fog condenses into water droplets and falls into troughs which flow to the village. The nets cost $200 each and cost nothing to maintain. [New Scientist, October 16, 1993, p.19]
The element having the lowest vapour pressure at room temperature is tungsten. The vapour pressure is so low that it cannot be measured even with the most sensitive instruments now available. One book suggests that the vapour pressure should be listed as “one atom per universe”. (Which brings one to ask how one might ever find the single atom to confirm the suggestion.)
Students are frequently taught that molecules are too small to see, but this is not necessarily true. A diamond is a single molecule, as is a sheet of glass and a piece of plastic wrap.
The fact that water is considered to be the solvent even when large amounts of another substance may dissolve in the water can be nicely illustrated with CaCl2•6H2O: at 20oC, 536 g of CaCl2•6H2O dissolve in 100 mL of water. Nevertheless, water is still the solvent in this case.
Gravity separation in the form of gold panning was a favorite method on the Tulameen River (between Merritt and Princeton B.C.) in the 1800’s. Since gold is much denser (density about 19.3 g/mL) than most rocks and gravel (density about 3 to 4.5 g/mL), the less dense rock is easily removed. The Tulameen also has large amounts of magnetite rock (density = 5.2 g/mL) which remains at the bottom of the gold pan with the gold. Miners also found small amounts of another very dense “rock” that they discarded since it wasn’t the gold for which they were looking. Later prospectors eagerly panned worked-out claims when they found that this very dense “rock” was pure platinum metal! To this day, the gravels of the Tulameen River (near the ghost town of Granite Creek) can be panned to give small amounts of gold and platinum and large amounts of magnetite.
Hand separation was the favorite method early explorers used to get rich in Burma and South Africa. The first explorers in Burma found that the indigenous people had many pretty red rocks that, on closer inspection, turned out to be rubies! When asked where they found the red rocks, the inhabitants pointed to the local streams which had numerous rubies sitting in shallow water, waiting to be picked up. A similar situation occurred on some South African beaches, where very early explorers reportedly found loose diamonds in the sand by the ocean.
In the late 1800’s “cloud seeding” was done by “rainmakers” to cause water vapour to separate out as ice crystals from the cold gas solution (air plus water vapour) existing in clouds. Silver iodide was used because it has a hexagonal crystal structure similar to ice crystals. Since the prairie air frequently had little dust or other contaminants in it at cloud-level altitudes, there were no “nucleation” points to provide a template to start growing ice crystals. The silver iodide formed the necessary nucleation points and as the ice crystals descended to earth they turned to rain. Because silver iodide was expensive to make, townsfolk had to pay ahead of time so that the rainmakers could purchase the necessary silver and other chemicals needed to make rockets to propel the silver iodide into the atmosphere before exploding into tiny “cloud seeding” particles.
An analogy for paper chromatography is a group of children at one end of a street being called by their mothers from the other end of the street. Along the way, some dawdle and eventually stop as they are attracted to candy stores, toy stores and so on. Some of the children are very obedient and run directly to their mothers, undistracted the attractions along the way.
Microwave ovens use a microwave frequency that strongly affects water molecules. An interesting way to destroy a microwave oven is to place very cold ice cubes on a cold plate and try to warm the ice in the oven. Since the water molecules in ice do not vibrate at the frequency used in most microwave ovens, the energy is not absorbed by the ice cubes. The net result is the same as turning on the oven when it is empty – which is NOT recommended since the oven can be ruined if operated when empty.
Infrared spectroscopy is routinely used to quickly identify samples of illegal drugs. The spectrum of the suspect substance can be obtained in a few minutes and quickly compared to the spectra of known drugs. The interesting thing about using this method is that if the drug is disguised by being mixed with other substances, the presence of the drug is still easy to spot. The technician simply looks for particular peaks in the spectrum and can ignore the peaks from contaminants.
Some textbooks have stated that glass is just a fluid that flows EXTREMELY slowly. Evidence of the ‘fact” that glass flows slowly is that glass in very old church windows is thicker at the bottom and thinner at the top. Not only that, glass rods sag after a few years if they are stored horizontally, supported only at their ends. There is only one problem with these facts: none of them are true! It is true that glass does not have a definite, repeating structure such as a crystal has, but glass is a true solid. It has randomly oriented bonds holding it together, but is a solid nevertheless. Sensitive strain gauges have shown conclusively that glass does not sag. What about the church windows that are thicker at the bottom and thinner at the top? Well, medieval glassblowers took a large hot blob of glass and spun it in a large furnace, in the same way pizza dough is sometimes spun in the air. The result is a large circle of thin glass. A cross-section through the glass is shown below.
Notice how the glass is thinner toward the middle and thicker toward the outer edge. The best, thinner glass was cut from the center and lower quality glass was cut from the thicker outer edge. Medieval craftsman left records telling us they deliberately placed the glass in windows with the thinner part upwards because they thought it looked better that way. Interestingly, people who have removed panes of old glass as part of a church restoration project have noted that sometimes the old glass was mistakenly installed with the thicker part upwards. Finding the thicker part on top is in line with the fact that glass does not flow or sag in a few hundred years.
Natural crystals of aluminum oxide are called corundum, emery, sapphire or ruby, depending on the impurities present. Although pure aluminum oxide crystals are transparent and colourless, the presence of various impurities give the gray to black colour of corundum and emery, chromium impurities produce rubies and a combination of iron and chromium impurities produce sapphires. By melting pure aluminum oxide and adding various impurities, scientists can now routinely make rubies and sapphires that weigh several hundred kilograms.
In the 1960’s the BC Forest Products lab at UBC would use a crane to lower an entire tree into a huge “blender” and then use solvent extraction to obtain various solutions for study. It was not unusual to add several thousand liters of some solvent such as alcohol and then stir for several hours to extract whichever chemicals would enter the alcohol solution. (The alcohol was later recovered by distillation.) After numerous subsequent extractions and separations of the original mixture, thin layer chromatography would be used to detect chemicals that might be present to the extent of a few micrograms per tree.
Unit IV: Inorganic Nomenclature
Aluminum was once considered to be a precious metal. In 1855, Emperor Napoleon III of France led one of the most powerful nations on earth. He was the chief financial backer of a French chemist who had created a refining plant to make small amounts of aluminum, and under his leadership France was now the world’s leading producer of this metal, which was more precious than gold and platinum. In fact, a one-kilogram bar of this metal had just been placed on display at the Paris Exposition, exciting all those who were privileged to see it. To display his wealth, the Emperor arranged to have a set of aluminum plates made which were used to entertain visiting ambassadors and heads of states. Aluminum remained as a precious metal until 1886, when two French and American chemists simultaneously discovered a way to produce aluminum on a large scale by passing electricity through a melted aluminum ore called bauxite. (Incidentally, how precious would gold be if it were more common and cheaper than iron?)
Lead, Pb, gets its symbol from the latin name “plumbum” (from which we get the word “plumbing”). Sewer pipes used to be made of lead tubes because lead was easy to make into sheets and tubes and because lead does not corrode easily. The Roman nobility also used lead pipes to get fresh water into their houses – which gave rise to an insidious poisoning problem that was aggravated by another tradition of Roman nobility: they liked sweet wine but had no sugar. What they did have was “sugar of lead” or lead(II) acetate, a sweet-tasting compound that they used to add to sweeten their wine. The resulting doses of lead were enormous: analysis of the bones of Roman nobles shows a lead content that is far in excess of that which would kill a present-day person – the Romans were very tough! It is interesting to speculate about the fact that the leaders of the Roman armies were drawn from the ranks of the nobility and what affect the heavy doses of lead had on their military abilities.
Silver metal is a known bactericide. The ancient Phoenicians kept drinking water pure on long voyages by keeping it in silver vessels. The slight solubility of silver in water, in the parts per billion range, is sufficient to kill typhoid bacteria and fecal bacteria. [Chem 13 News, September 1993, p. 5]
One troy ounce of gold (31.1 g) can be hammered into a sheet covering more than 100 square feet (more than 9.3 square meters). (Future question for Unit V: If gold has a density of 19.3 g/mL = 19.3 g/cm3, how many atoms thick is this sheet?) [Chem 13 News, April 1991, p.15]
Helium is named after the Greek word for the sun (Helios) because helium was first found from the light spectrum from the sun.
Iron is very common in compounds such as iron oxide, iron carbonate and iron silicate, but is very rare as pure native iron. An interesting thing about native iron is that it is soft enough to be cut with a knife. Most native iron on earth is the result of a meteor impact but a few locations in Greenland, Germany, Ontario and Lillooet BC are non-meteoric sources of this rare mineral. Native iron is rare because it quickly reacts with air and water to form iron compounds (rusting), and therefore native iron only occurs under special conditions that prevent water and air reaching and reacting with the metal, and in reducing conditions. (Since microscopic cracks in solid rock extend thousands of feet below the surface, water and air can usually react with, and change, minerals in the top few thousand feet of the earth’s surface.)
Native copper is not as rare as native iron but is equally interesting. Afton Mine, just to the west of Kamloops, contained deposits of native copper which formed in the cracks of the surrounding rock. The Keweenaw Peninsula in northern Michigan is the site of a massive lava flow that spewed out liquid copper metal over a 200 mile long distance. Some of the melted copper sank into the underlying ground, solidifying and completely encasing the underlying pebbles and rocks. One chunk of copper was bigger than a two-story house! Because the big chunks of copper were so difficult to break up, melt, or mine, the area was eventually left as a park.
Mercury is a liquid metal found, on rare occasions, in little puddles and globules in the cavities of rocks containing cinnabar (mercury sulphide). The liquid mercury is usually created as a result of the surrounding rocks having been heated to high temperatures. In a few locations, the size of these puddles has exceeded several tons! There are a few locations near Lillooet BC where native mercury can be found.
Selenium and tellurium are elements that require extreme care in handling. Accidental contact with these elements causes some of the elements to absorb through the skin and for a long time thereafter the careless experimenter’s breath and sweat stink of rotten cabbage and rotten garlic.
White antimony oxide used to be favoured as a face powder by young women in the late 1700’s (it gave them a pale look which implied that they were ladies of leisure and were not like “common” women who had to labour outside in the sun). During the same period, it was also fashionable to go to the city of Bath and “take the bath” with a gentleman friend. (This was not a risqué activity; bathing costumes covered a young woman from the top of her neck to her ankles and wrists.) Unfortunately for young women who “took the bath” AND used antimony oxide as a face powder, the hot springs which fed the baths contained relatively large amounts of hydrogen sulphide. As the dissolved hydrogen sulphide was boiled out of the hot waters, a chemical reaction occurred between the antimony oxide and the sulphide ion, producing antimony sulphide. Since antimony sulphide is black, the young woman’s face started to turn light grey, then dark grey and finally black. If the young lady’s escort tried to rub off the face powder, the reaction immediately produced a black compound which adhered strongly to skin and pores.
Virtually all chromates are bright yellow, and lead chromate is no exception. Lead chromate used to be the preferred pigment for making bright yellow paint but was eventually banned from use in paints when it was found that many young children (who chew on everything when they are teething) had received large doses of lead from chewing on lead-based paint. These unfortunate children were left with permanent massive mental impairment.
Phosphoric acid, found in many soft drinks (especially colas), is able to slowly dissolve glass. Hence, cola beverages in glass bottles have a definite “shelf life” because the phosphoric acid etches the glass by removing the glass surface. Cola drinkers might want to think about what that phosphoric acid is doing to the enamel of their teeth. Experimenters using glass stopcocks occasionally dipped “frozen” stopcocks into cola to eat away a small amount of the glass and free the two parts of the stopcock.
A mineral is a naturally-occurring inorganic substance with a definite chemical composition. For example, the most common mineral on earth is water. Huh? Water is a mineral? Yup! But I thought minerals were things that miners dug out of the ground! Well, that’s partly true but ice and liquid water ARE naturally-occurring inorganic substances with a definite composition - H2O - so they are different forms of the mineral “water”.
Gypsum [calcium sulphate dihydrate or CaSO4•2H2O] is a very common mineral found in many places in BC. Typically, gypsum crystals are colourless and transparent with the following shape.
Some minerals are difficult to find and require long hikes into remote locations. Not gypsum! Many mud-filled roadside drainage ditches contain beautiful gypsum crystals up to 7 cm long. Such crystals grow in the ditches around farmer’s fields near the towns of Swift Current and Willow Creek in Alberta. The ground is rich in calcium and sulphate ions, and as the water in the ditches evaporates the crystals form because they have a low solubility in water. In Saudi Arabia, beautiful black gypsum crystals (black because small amounts of powdered black sand impurities are included in the crystals) are found in the sand near the seashore. The local legend is that these crystals only form where a camel has urinated, but the truth is that the crystals form for the same reason the crystals in Alberta grow in ditches – evaporation of water containing calcium and sulphate ions allows gypsum crystals to grow.
Is a school principal a boron leader?
- What doctors do: helium
- What undertakers do: barium
- The outside of a bovine: oxide
- Mailed a report card: centigrade
- What the Greeks said to the Trojans: hydrogen
- A trip after dark: nitride
- An element beloved by nieces and nephews: auntie-mony
[J.Chem.Ed., Vol.78 No.4, p.466]
A student at Eagle Rock Junior High won first prize at the Greater Idaho Falls Science Fair. He was attempting to show how conditioned we have become to alarmists practicing junk science and spreading fear of everything in our environment. In his project he urged people to sign a petition demanding strict control or total elimination of the chemical dihydrogen monoxide for plenty of good reasons, including:
- it can cause excessive sweating and vomiting
- it is a major component in acid rain
- it can cause severe burns in its gaseous state.
- accidental inhalation can kill you.
- it contributes to erosion.
- It decreases effectiveness of automobile breaks.
- It has been found in tumors of terminal cancer patients.
He asked 50 people if they supported a ban of the chemical. Forty-three said “yes”, six were undecided, and only one knew that the chemical was water. The title of his prize-winning project was “How Gullible Are We?”. He felt the conclusion was obvious.
Unit V: The Mole Concept
A bloodhound can detect the smell of a single molecule of butanoic acid (C4H8O2), a compound that smells like distilled gym sneakers and is produced by all human feet. When a person walks, the butanoic acid is absorbed by the soles of their shoes, so that every step leaves about 105 molecules of butanoic acid on the ground. Students could be asked what mass of butanoic acid we leave behind with every step we take.
Part of the problem in dealing with the mole is that fact that a mole of molecules represents an unimaginably large number of unimaginably small particles. Try using the “dozen” as an analogy for Avogadro’s Number. Then one would have a “dozen mass”, a “dozen volume” and the “dozen number”. (The experimentally-measured “dozen mass” of extra large eggs is about 828 g. The experimentally-measured “dozen volume” of extra large eggs is about 708 mL. The “dozen number” is 12. If one wanted to know the mass of an individual extra large egg, dividing the dozen mass by the dozen number gives a mass of 69 g.)
When the mole concept is just being introduced, plunk down a quarter (or a loonie if you feel rich) and state that the quarter/loonie can be claimed by the first person to bring one mole of water to the teacher. After a few seconds thought, some kids will see if there is a balance handy (usually there isn’t) and then realize that the density of water is 1 g/mL and run to get a graduated cylinder and fill it with 18 mL of water.
An oldie but goodie question for a class: The total mass of the water in the oceans, rivers, lakes and as atmospheric vapour is about 1.66 x 1024 g. If Julius Caesar’s body contained 54 kg of water (66% of a healthy human body is water; assuming Caesar had a mass of 82 kg (180 pounds)) just before he was cremated, and if the water in Caesar’s body has now thoroughly mixed with the rest of the waters on earth, how many water molecules that used to be part of Julius Caesar will you consume when you drink 250 mL of water? (answer = 27,000 molecules)
A discussion question for a class: A 10 g crystal of calcite (CaCO3) typically takes about 100 years to grow. How many molecules of CaCO3 are added to the crystal each second? (answer = 1.9 x 1013 molecules/s)Emphasize how fast molecules must be moving in solution and how many effective collisions by the building-block ions must take place in order that the crystal grows at such a slow rate.
Mole remover = any student who removes himself mentally and/or physically from the classroom during discussions of moles [Chem 13 News, September 1991, p.7]
Unit VI: Chemical Reactions
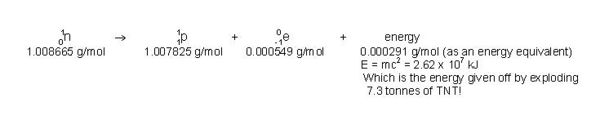
Strictly speaking, physicists now combine the laws of conservation of mass and conservation of energy into a law of conservation of mass/energy. (For the purposes of the low energies involved in high school chemistry, however, the individual conservation laws are said to hold exactly.) The reason for this combination stems from Einstein’s equation, E = m•c2, which states that a gain or loss of mass is accompanied by a proportional gain or loss of energy. For example, when one mole of deuterium and one mole of tritium react together in a fusion reaction, the products are one mole of He-4, one mole of neutrons and energy. Since the total mass of the products is 18.8 mg less than that of the reactants, the mass equivalent of 18.8 mg of matter is released in the reaction, 1.69 x 109 kJ,which is equivalent to the energy released by the explosion of 470 tonnes of TNT!
During the 1982 Falklands War between Great Britain and Argentina, Argentina launched an Exocet missile against the British destroyer HMS Sheffield. The missile came in low and struck the upper part of the ship called the “superstructure”. Initially the fire that resulted was thought to be easily contained but as soon as seawater was poured on the fire it rapidly became out of control. The reason for this sudden flare up was the fact that the superstructure was made of an aluminum alloy, which, like all aluminum, ignites relatively easily and burns extremely fiercely when water is added to burning aluminum. Eventually, the destroyer sank. This is an interesting story to accompany the addition of a few drops of water to a mixture of powdered aluminum and powdered iodine in a fume hood (bursts into flame and produces aluminum iodide in a synthesis reaction).
An analogy for a single replacement reaction is a situation where Robert and Roberta go to a formal dance. Harry “cuts in” and waltzes away with Roberta, leaving Robert standing alone. Note that if a student tries to predict a reaction as: (Metal #1)+(Nonmetal)– + (Metal #2) ===> (Metal #1)+(Metal #2)– + (Nonmetal) this implies that Harry cuts in and waltzes away with Robert, leaving a very confused Roberta standing alone. (Care how you treat this latter situation in today’s politically-correct atmosphere.)
An analogy for double replacement then logically follows the above analogy: Robert and Roberta go to the dance with Harry and Harriet. Both couples have a spat and break up but Robert starts to dance with Harriet while Harry starts to dance with Roberta.
One single idea can tie together all the concepts associated with reaction energies. (Tell students to concentrate on exothermic reactions; use the opposite concepts for endothermic once exothermic is memorized.) The central idea: “Exo is a loser!” (apologies if this seems politically incorrect). Exo loses energy, ∆H is negative, the system loses energy to the surroundings, reactants go downhill to get to products.
If a student places a beaker full of cold water on a wire gauze on a ring stand and starts a Bunsen burner below the beaker, the outside of the beaker gets a thin coating of condensed water on the outside. The question is: where did the water come from? The source of the water is NOT water vapour from the air, since an identical beaker full of cold water sitting on the desk does not show the same condensation. A little thought shows that the source of the water is the combustion of the burner gas. Since the beaker initially is cold, the water vapour produced by the combustion condenses until the outside gets hot enough to vaporize the condensate again. [Chem 13 News, November 2000, p. 3]
An interesting example of an exothermic reaction is the setting of epoxy glue. If some two-part 5-minute epoxy is made up and allowed to set between two pieces of paper, the paper becomes warm as the epoxy sets. Because epoxy forms bonds between monomer units, the formation of the bonds is accompanied by heat production.
Students frequently don’t understand the difference between “heat” and “temperature”, so grab their attention by pointing out to them that they can freeze to death at one million degrees Celcius. ‘Temperature’ is a measurement of the average kinetic energy of the molecules making up a substance. ‘Heat’ is the transfer of energy from something that has a higher temperature to something that has a lower temperature. In simpler terms, temperature is a measurement that tells us how much energy an SINGLE average molecule contains, while heat is the total amount of energy that ALL the molecules present can deliver. Let’s look at an example. Pretend you have boiled one hundred litres of water. That water has a temperature of 100oC. Next, pretend you take an eyedropper, fill it with boiling water and let exactly one drop of boiling water fall onto the palm of your hand. (Oh, the pain, the agony!) You would not feel very much because there is not much heat in a single drop of water, even if it is at 100oC. On the other hand (pun intended), if you threatened to pour the entire 100 L of boiling water over a student’s hand, you wouldn’t want to imagine the pain and damage that would occur. Both the single drop of boiling water and the 100 L of water have the same temperature: 100oC. This means the average energy of the water molecules in the single drop and the 100 L was identical. But, the single drop of water transfers much, much less heat energy to your hand than is transferred by the 100 L of water. So … how can you freeze at high temperatures? Well, the outer fringes of our sun (called the corona) has a temperature of about 1,000,000oC. However, the corona contains so little gas(*) that it is practically a vacuum, in spite of the fact that each of the very few gas particles present have high individual energies. Let’s pretend again. You are in a spacesuit, directly exposed to the near-vacuum of the sun’s corona, with your space ship between you and the sun so as to prevent you from being ‘cooked’ by direct radiation from the sun. If the heating unit on your spacesuit fails, you will start to freeze in spite of being surrounded by a gas at one million degrees. Why? Well, each gas particle may have a huge energy as far as the gas is concerned, but atomic particles are extremely small and we are very large, so even if one of the gas particles transfers all its energy to our spacesuit we wouldn’t notice the energy it gives us. The ‘few and far between’ gas particles in the corona will transfer their little bit of heat energy to you when they hit your suit, but because your spacesuit’s heater has stopped you would be losing body heat faster than heat was coming in. The overall result is that you would freeze because you were losing more heat than you were gaining. As a final example, the gas (actually, it is a plasma) inside a long fluorescent light has a temperature of about one million degrees when the light is operating. If you have touched the tube of an operating fluorescent bulb, you know that the bulb does not feel very hot. The inside of the bulb is almost a vacuum and although the few individual gas particles have high individual energies, the total amount of heat energy they deliver to your hand is very small.
- (*) Technically speaking, the particles in the corona are called a plasma because all the electrons have been stripped off the atoms, leaving a collection of free electrons and naked atomic nuclei. However, we will continue to call it a gas to make our explanation easier to understand.
An analogy to the fact that ∆H for exothermic reactions is negative comes from standard accounting practice: gains are reported in financial statements and losses are recorded as negative numbers (actually, losses are usually printed in parentheses and are interpreted as negative numbers. For example, a loss of $50 is printed as (50), and is interpreted as “-50”).
Students may wonder what causes the wispy white vapour that arises from a just-opened bottle or can of soda. This is a nice example of an endothermic process. When the container is opened the pressurized gases rapidly expand outward and take their kinetic energy with them. (The attractive forces between the gas molecules are also broken, which requires kinetic energy to be absorbed by the molecules and lowers the temperature.) This loss of kinetic energy leaves a region in the neck of the bottle (or airspace of the can) that is hugely deficient in kinetic energy and so plunges in temperature to about -33oC. Water vapour then condenses into visible floating droplets that rise as a small cloud. [Kitchener-Waterloo Record, July 24, 1993, p.A5]
Experimuck = the remains left in a test tube after an experiment is done [Chem 13 News, September 1991, p.7]
What is in hot packs and cold packs?
Hot packs:Most “single usage” hot packs work in the following way. A plastic pouch contains water and a second, sealed pouch with a dry chemical inside. When the outer pouch is firmly squeezed and then shaken, the inner pouch breaks and the dry chemical mixes with water. The dry chemical, which is usually calcium chloride or magnesium sulphate, bonds with the water and creates an exothermic reaction, producing a great deal of heat. The water in the outer plastic pouch is usually contained in an inexpensive “gel” (made of cellulose, colouring, salt and a preservative) that retains heat and slows the reaction. By slowing the reaction, the temperature is prevented from becoming too high, causing burns, while extending the length of time that the reaction provides heat. Calcium chloride and magnesium sulphate are used because they are inexpensive and contain calcium ions and magnesium ions. Calcium and magnesium ions are very small, so that they can get very close to the water molecules and attract them strongly, giving off lots of heat. In addition, these ions have a large electric charge which attracts the water molecules very strongly and again increases the amount of heat given off. [Calcium chloride is available from hardware and building supply stores as a “de-icer” and is thrown on sidewalks and driveways in the winter to help melt ice. The calcium chloride bonds strongly to the water molecules in the ice and produce sufficient heat to melt the ice and keep it melted. Magnesium sulphate is commonly called “Epsom Salts” and is used as a laxative because it has a strong tendency to absorb water from your body and make bowel movements easier. Amateur mineral collectors may be interested in knowing that the mud at the bottom of Venables Lake, southwest of Ashcroft, is full of large and beautiful magnesium sulphate crystals called epsomite!]
A different type of hot pack is reusable and contains sodium acetate crystals and a tiny amount of water. When the plastic pouch containing the sodium acetate is placed in boiling water or a microwave, the sodium acetate absorbs heat and melts. Sodium acetate has a very interesting property that is shared by only a few other solid chemicals: when it is heated and allowed to cool, it remains as a liquid! Now think … if adding heat causes a solid chemical to become a liquid, what happens if the reverse situation occurs and the liquid becomes a solid again? That’s right! Heat is given off. Because sodium acetate has difficulty solidifying after it has been melted and cooled, a little metal “clicker” is usually placed inside the plastic pouch before it is sealed. Clicking the piece of metal creates a disturbance in the liquid and causes the cool, liquid sodium acetate to quickly solidify and give off heat as it crystallizes. Heat is given off for about 20 minutes. After being used, the plastic pouch can be reheated, so as to be ready for the next usage.
A third type of hot pack consists of a porous plastic bag containing powdered iron,flakes of magnesiummetal and salt. When salt water is poured on top of the porous bag, enough heat is produced (by a complex chemical reaction) to cook a meal in 14 minutes. These self-heating meals were originally used by the US Army and are now commercially available. You can make a hot pack by placing about a spoonful of calcium chloride de-icer in a resealable plastic bag and adding about a cup of water. The more calcium chloride added, the higher the temperature becomes, so be careful not to create a high temperature that causes burns.
Cold packs:The bonding of water molecules to other molecules or ions produces heat in an exothermic reaction. Another example of an exothermic reaction occurs when a positive ion and a negative ion are attracted together and join. If the reverse of this process occurs, energy must be added to make the positive and negative ions pull apart from each other. Because the surroundings have to give up some of their heat in order for the reaction to absorb heat, the surroundings get cooler. The most common substance used in cold packs is ammonium nitrate. This chemical is inexpensive, dissolves easily in water and absorbs a large amount of heat from its surroundings during the dissolving process. A cold pack made from ammonium nitrate consists of a plastic pouch containing water and a separate pouch containing solid ammonium nitrate. As with hot packs, the cold pack is squeezed and shaken to break the inner pouch and mix the contents. The pouch gets very cold as the solid chemical dissolves and takes heat out of the water, pouch and the surroundings (you!). As with hot packs, a cellulose gel is frequently used to extend the length of time that cooling is felt and prevent the mixture from becoming too cold. Without the presence of such a gel, a cold pack made from ammonium nitrate could get cold enough to freeze flesh.
A somewhat different kind of cold pack, created for the US Army by a company called Dual Ice, is made from ammonium chloride, urea and a special mixture called a buffer, which holds the pH steady. When the inner seals of the pouch are broken and the ingredients mixed, the temperature quickly plummets and provides cooling. The advantage of this cold pack is that the used cold pack is reusable: it can be placed in a freezer for a few hours, forming a slush that provides cooling for 30 minutes. You can make an inexpensive cold pack at home by mixing 1/3 of a cup of rubbing alcohol (poisonous!) with 2/3 of a cup of water in a re-sealable plastic bag. When placed in a freezer for a few hours, the mixture forms a slush and is ready for use. This home-made cold pack can be used and refrozen several times.
Unit VII: Calculations Involving Chemical Reactions (Stoichiometry)
Marine biologists have shown that the oceans are nutrient-rich but are iron-poor. In fact, iron is the limiting reactant for algae growth. In 1995, scientists “seeded” three 60 square kilometer patches of ocean west of the Galapagos Islands with iron. As a result, algae growth “exploded” to the extent that half a tonne of iron caused 100 tonnes of carbon dioxide to be removed from the atmosphere. The leader of the experiment has argued that the high concentrations of iron reaching the oceans during the last ice age was responsible for removing vast amounts of carbon dioxide from the atmosphere and keeping the planet cool. Supporting evidence comes from high concentrations of iron-containing dust in ice laid down during the last ice age and a corresponding high concentration of carbon on sea beds (from algae which flourish and then sink to the ocean floor). [New Scientist, October 12, 1996, p.4
In the 1950’s it was not uncommon for kids to react aluminum foil with sodium hydroxide and capture the aluminum produced in a modified plastic bag of the type used to protect dry cleaning. The aluminum foil was the limiting reactant.
When the bag was properly sealed, the kids attached a slow-burning fuse to the bag at night, lit the fuse and let the bag quickly ascend up into the air. At an altitude of several thousand feet, the bag erupted into a fireball as the hydrogen ignited (It burned quickly but didn’t explode since bag contained hydrogen rather than a hydrogen-oxygen mixture). It was not unusual to have people phoning radio stations and the airport to report the bright burst of light in the air. One wonders if some UFO reports were also started.
Most Chemistry professors perform their research with chemical reactions involving no more than one gram of reactants, and sometimes use milligram or microgram amounts. Hence, it was a “whole new ball game” for a retired Chemistry professor who volunteered to make potassium sodium tartrate tetrahydrate (Rochelle salt) for the BIG Little Science Center in Kamloops. Why? They needed 132 kg of Rochelle salt. If the chemical reaction for producing potassium sodium tartrate tetrahydrate from potassium hydrogen tartrate (“cream of tartar”) is:
what mass of cream of tartar and sodium carbonate are required to make 132 kg of potassium sodium tartrate tetrahydrate? (The retired Chem prof? He had to re-think much of the equipment he needed. Hot plates were replaced by barbecues, reaction flasks were replaced by 20 L barrels and test tubes by 3 L plastic trays.)
Chemical technicians working for mining companies use stoichiometry on an every-day basis. As new ore veins are opened and old ones start to be “played out”, the technicians take typical rock and ore samples coming from the day’s production, weigh the sample and carry out an extraction process to end up with a pure sample of the metal being produced by the mine. Once the mass of the pure metal is known, the technician calculates the percentage of the desired metal contained in the original sample and helps the mining engineer decide whether the ore vein is commercially profitable.
Scaling up a basic recipe, say a chocolate cake to serve 6, such that the recipe will serve 4000 crew on an aircraft carrier, is an example of practical stoichiometry.
Unit VIII: Atoms and the Periodic Table
Science does not have all the answers, and in fact does not even know what many of the most fundamental things are. For example: we do not know what time is except that it is a property of the expanding universe; we do not know what mass is, although theories have been proposed that mass is created by a particular as-yet-unfound subatomic particle; we do not know what electric charge is nor why the electric charge on an electron is exactly equal and opposite in sign to the charge on a proton (which is thought to be unrelated to an electron).
Masurium: the element that wasn’t. Mendeleev knew that there were still several undiscovered elements when he published his first periodic table in 1869. He left a space for element 43 and in 1872 predicted its properties and called it eka-manganese. In 1925, German scientists discovered a new element which they named “Masurium” and gave the symbol “Ma”. For several years the existence of masurium was debated between scientists of various nations and its existence eventually was disproved by X-ray studies. Element 43 was actually discovered in 1937 and was later named “Technecium” because it was the first element produced by modern technology. Because of the secrecy surrounding nuclear research during the second world war, periodic tables proudly showing Ma as the symbol for element 43 were published and placed in school and university classrooms as late as 1943.
When Mendeleev published his periodic table in 1869, he found that some elements had properties that didn’t seem to fit in with his predictions. As a result, he took the bold step of telling experimenters that their results were probably in error and should be re-determined. For example, Mendeleev predicted that the density of the unknown element eka-aluminum, later to be identified as gallium, (“eka” is Sanskrit for “one”) should be 5.94 g/mL. When gallium was discovered in 1875, the density was found to be 4.7 g/mL and the discoverer stated that Mendeleev was wrong. Mendeleev then insisted that the density be re-measured and when this was done, the new value for the density was found to be almost exactly as Mendeleev had predicted.
Students are usually fascinated by nuclear physics and the sometimes-impossible-to-comprehend behaviour and properties of nuclear particles. For example, many nuclear reactions produce “neutrinos” – particles which have no charge and no mass and only have one property other than energy: they have “spin” (ask students to visualize a particle which can be described as nothing, spinning on its axis! (OK, some physicists now believe that neutrinos may possess an incredibly tiny mass, probably thousands of times less than that of an electron.) To make the situation more bizarre, neutrinos can have either clockwise spin or anti-clockwise spin and come in more than one variety, including anti-neutrinos.
One of the proofs that the neutron is NOT a true fundamental particle is the fact that if a collection of neutrons is knocked out of some atoms, half of the neutrons will decay to a proton and an electron after 10.3 minutes:
Some alchemists claimed to have turned base metals (for example, lead) into gold. However, modern nuclear physicists have actually made gold from lead. The catch to this gold-making process is that the gold that was produced was highly radioactive, and a very tiny amount was made. The cost of the radioactive gold was in excess of one billion dollars per gram, which makes the process rather impractical. The energy involved in making lead turn into gold was enormous. If an alchemist had succeeded in producing even one gram of gold it is likely that someone would have recorded a side effect of the energy requirement: the gram of gold would have required the burning of most of the forests produced over a hundred year period in all of Europe and Asia.
The earth’s oxygen supply consists of 99.76 % 16O, 0.04 % 17O and 0.20 % 18O. Measurement of the value of the ratio 18O/16O can be used to calculate the average ocean temperature in the geological past (millions, tens of millions and hundreds of millions of years ago). When water evaporates from the oceans, H216O molecules are lighter than those of H218O and hence evaporate to a somewhat greater extent. Hence, H216O accumulates in the atmosphere to a greater extent. However, when rain forms from the water vapour, the heavier isotopes precipitate preferentially. As a result, if ocean temperature are cool the water vapour that makes it to the north and south polar regions is especially concentrated in lighter isotopes and ice formed at this point has a lower 18O/16O ratio. On the other hand, small ocean-dwelling critters called “foraminifera” form CaCO3 shells made from the oxygen in the water molecules remaining in the oceans. When the ocean temperatures are lower, the ocean is preferentially enriched in H218O and hence foraminifera shells contain higher 18O/16O ratios. By calibrating the exact 18O/16O ratios found at different temperatures, scientists were able to tell what the average ocean temperatures were in the distant past using either ice core samples (useful up to about 500,000 years ago) or shell samples in rocks (useful up to several hundred million years ago).
When dealing with the colours of the rainbow and light emitted as atoms relax from higher to lower energy states, the mnemonic ROYGBIV is actually a holdover from 17th century mysticism. When Isaac Newton discovered how to use a prism to separate white light into a rainbow of colours, he insisted there were 7 pure colours in the spectrum, in spite of the fact that any observer can only see 6 colours (red, orange, yellow, green, blue and violet). Newton insisted that a 7th colour, indigo, was present because according to his mystic beliefs 7 was a special number and this special number must manifest itself in nature. Generations of students have been frustrated by trying to convince themselves that they could “see” indigo as a pure colour in the rainbow, different from violet.
Arnold Sommerfeld, a German physicist, realized that the top diagram on page 151 of the Chem 11 Workbook resembled something he had seen before. After a quick calculation, he realized that the spectrum was a perfect fit to a 1/X2 relationship. Nature was based on math!
Albert Einstein was playing his violin in a duet with Werner Heisenberg (the discoverer of the Heisenberg Uncertainty Principle), who was accompanying him on the piano. After a while Heisenberg slammed his hands down on the keys and said: “It’s one, two, one, two, Einstein! Can’t you count?” [New Scientist, December 25, 1993, p. 41]
When a higher energy electron jumps to a lower energy level, the electron never possesses an intermediate energy between the energies of the upper and lower levels. A visual analogy is to consider putting grandma’s best china vase on one shelf of a set of shelves: you can put the vase on an upper shelf or on the next shelf down but not on a shelf between the two. Incidentally, if an electron is far from the nucleus in a high-energy orbital and jumps to a low-energy orbital close to the nucleus, the electron not only never has an intermediate energy, it never occupies an intermediate position. It simply was “there” and now is “here” and was never “in between”.
The noble gases were originally thought to be completely unreactive, until 1962 when Professor Neil Bartlett prepared XePtF6 at UBC. (One unfortunate property of this chemical was its unpredictable tendency to explode, as one of Barlett’s graduate students found while taking a sample out of the lab to get it analyzed. The student survived but the glass door he was going through at the time did not.) After this breakthrough, numerous compounds of the noble gases were made.
When discussing periodic trends, it can be mentioned that hydrogren telluride (H2Te) smells like rotten garlic, hydrogren selenide (H2Se) has a smell like rotten cabbage and hydrogen sulphide (H2S) smells like rotten eggs. This leads one to ask what smell would we predict for hydrogen oxide (H2O). Although we say water has no smell, other animals can definitely smell water from large distances. Students might like to suggest why we can’t smell water but other animals can. Iron has a body-centered cubic crystal structure in which each atom is at the center of a cube made from 8 other iron atoms. Because iron joins to other iron atoms with bonds that involve d-orbitals, iron metal is quite malleable because the d-orbitals point in multiple directions and can accommodate many different bonding directions. When carbon atoms are introduced into iron, making steel, the carbon atoms are highly directional in their bonding, effectively “locking” the adjacent iron atoms in place and making the resulting steel harder and less malleable.
Unit IX: Solution Chemistry
The hexagonal structure of ice crystals is an interesting opportunity to show how misleading questions can send our thought processes down the wrong path. Point out that a typical snowflake has six more-or-less identical “arms” and that each snowflake has a different appearance (although there are broad similarities among the various classes of snowflake shapes). Now the “zinger”: ask a class how one arm of a snowflake “knows” what shape it should have in order to be identical to the other arms on the snowflake. (Do they all have tiny cell-phones to keep in touch with each other? “OK gang, now let’s all grow a little thin arm for a bit and then expand slowly before getting smaller again. Synchronize watches. The operation starts … now!”) After a spirited discussion, hopefully someone (such as the teacher, if necessary) will point out that each arm is effectively a “time axis” with zero time (start of growth) at the center and time increasing when going out along each arm. When the snowflake falls through a part of the atmosphere richer than average in water vapour, the growth of all arms increases and when passing through water vapour-poor parts the growth rate slows for all arms. Hence, each arm is simply recording the identical conditions through which the snowflake falls.
Carbon tetrachloride was formerly used as a dry-cleaning solvent because it dissolves many organic compounds and easily gets sweat stains off the neck of clothes. Usage was discontinued when studies revealed that inhaling carbon tetrachloride fumes destroyed approximately one million brain cells with each inhalation.
It is a fact that women become intoxicated quicker and suffer worse consequences (including heart and liver damage and cancer) from consuming alcohol than men. The reason for this is that women have a greater percentage of body fat than men, resulting in lower amounts of polar aqueous fluids I n which polar alcohol can dissolve. Polar alcohol does not dissolve in non-polar body fat. Allowing for body weight and taking men and woman of equal physical fitness, alcohol reaches a higher concentration in women and hence has a greater effect. Another effect of the polar properties of alcohol: alcohol consumption increases the carcinogenic effect of cigarette smoking. Tar from cigarette smoke is nonpolar and does not dissolve readily in water and in polar aqueous saliva. The nonpolar “tail” of alcohol (CH3CH2 is nonpolar; only the OH end gives alcohol its polar properties) dissolves some of the tar lining the mouth, nose, throat and lungs of a smoker and transports the tar through membranes and into the smoker’s body, enhancing the carcinogenic effects of smoking. [J. Chem. Ed. April 2000, p. 475]
Are London forces just British military?
Is Be2+Ar2- a polar bear? [BC science teacher conference talk by Julius Sumner Miller, date unknown]
Unit X: Organic Chemistry
Chloroform, CHCl3 was used as an anaesthetic in the 1800’s and became a popular anaesthetic to administer for childbirth after it was administered to Queen Victoria for this purpose. Unfortunately, chloroform has one small(?) drawback: the amount of chloroform needed to produce anaesthesia is only slightly less than that needed to kill a person. Since many doctors using chloroform were relatively inexperienced with the anaesthetic, many deaths of otherwise-healthy people occurred and chloroform was soon replaced by other, safer anaesthetics.
Many common chemicals act as pheromones for animals and insects. For example:
- Acetic acid and propanoic acid are defense pheromones for ants
- Formic acid (methanoic acid) and 2-heptanone are alarm signals for ants
- Phenol is a sex attractant for the grass grub beetle
[J.Chem.Ed. Date and volume unknown]
Chloroethane in a spray can is used as a topical anaesthetic for sports injuries (when the chemical is sprayed from the can, its low boiling temperature, 12.3oC, causes it to evaporate rapidly and cool the affected part, giving rise to a numbing effect by freezing.
The document “Chem 11 Demos.doc” (at http://bctf.ca/bcscta/resources/hebden/hebden.htm) gives the directions for making six esters (Demo 11.X.7, “Esters as Natural Perfumes”). Some other possible esters and their odours are listed below. Teachers might consider having some students make sample for extra credit or add the synthesis to their repertoire. Note: no directions are given so caution must be exercised. Also, butyric acid and valeric acid are very stinky.
- Amyl benzoate (odour of ambergris)
- Amyl butyrate (fruity odour)
- Benzyl acetate (ethereal and fruity odour)
- Benzyl benzoate (slightly balsamic odour)
- Ethyl acetate (fruity odour)
- Ethyl benzoate (odour is similar to methyl benzoate, below, but is softer and more aromatic)
- Ethyl butyrate (resembles the odour of a rose)
- Ethyl cinnamate (balsamic and fruity odour)
- Ethyl formate (similar odour to ethyl acetate)
- Ethyl laurate (pleasant but not powerful odour)
- Ethyl nonylate (odour suggestive of roses)
- Ethyl phenylacetate (distinct odour of honey)
- Ethyl salicylate (much like methyl salicylate or wintergreen but finer)
- Ethyl valerate (flowery and fruity odour)
- Isobutyl acetate (odour of hyacinth and roses)
- Isobutyl benzoate (pleasant odour suggestive of lilies)
- Isobutyl phenylacetate (sweet odour, suggestive of sweet briar rose)
- Isobutyl salicylate (very sweet odour)
- Methyl anthranilate (odour resembles orange blossoms)
- Methyl benzoate [“niobe oil”] (strong, balsamic odour)
- Methyl cinnamate (heavy, fruity odour, suggestive of strawberries)
Extensive double bonding alternating with single bonds in a molecule (eg. C=C-C=C-C=C) causes the molecule to be coloured. Changing the number of double bonds changes the colour in a predictable way (the system of alternating double and single bonds is actually a tuned antenna). When bleach is added to such molecules, one or more of the double bonds are destroyed (the bleach reacts with the double bonds to create single bonds), changing the colour or even making it completely disappear.
Chemistry 12 Tidbits
Unit I: Reaction Kinetics
At the start of the year, Chemistry 12 students usually accept any statement made by the Chem teacher as if it were delivered on tablets on Mount Sinai. A true scientific mindset requires that scientists question everything until they have verified the probability that a statement is true. I found the following approach to be very effective in making students carefully analyze all my statements … Before they even opened their books, I told them that we were going to start the year by considering the rates of chemical reactions and then wrote the first equation in the Chem 12 Workbook on the blackboard: 2 N2(g) + 5 O2 (g) + 2 H2O(l) ===> 4 HNO3(l) + 121 kJ. I asked them if the equation was exothermic or endothermic and they agreed it was exo … (lulling them into my trap). Then I drew the diagram at the top of page 1, showing that the energy of an exothermic reaction starts somewhere and asked if the energy goes up or down from REACTANTS to PRODUCTS. They quickly agreed that the energy went down (docile complacency has now set in). I then stated that “it was obvious” that exothermic reactions occur spontaneously but endothermic reactions require heat to be added in order to occur. Again, the class would nod dumbly, accepting all my statement as pronouncements of some kind of ultimate truth … and now I sprung the trap! I asked the class how many of them had gone swimming during the summer holidays, or had at least taken a bath or shower. Looking confused, all hands went up. Then I would say to some of my former Chem 11 students, as I made a big show of peering at their hands and arms “You obviously had a wonderful plastic surgeon! I didn’t think you were so suicidal as to go into water, but at least the plastic surgeons were very skilled!” Students were now looking around as if to try and escape and notify the principal that I had completely lost my mind. I quickly asked where they could find a cheap and plentiful source of oxygen gas. “The air”, they responded. Where can you find lots of nitrogen gas? “The air”, they responded again. And where can you find large amount of liquid water? “Lakes, rivers and the oceans”, they responded. Now I pointed out that they had agreed with my statement that it was obvious that exothermic reactions were spontaneous, and therefore oxygen, nitrogen and water will spontaneously combine to make nitric acid. Surely, anyone who dives into nitric acid will need plastic surgery to heal the chemical burns to their body (if they survive) and it is truly suicidal to plunge into nitric acid! Pained looks on student faces ... they know they have been “had”! After discussing the idea that some sort of “brick wall” must prevent the nitric acid formation since the oceans have existed for billions of years and contain negligible amounts of nitric acid, I concluded: whenever I, or anyone else, says “isn’t it obvious that …” the students MUST think very carefully for themselves and start by saying “I will not accept a statement by an authority until I have thought about the statement for myself”. I called the incorrect acceptance of a statement “going down the garden path” and warned them that I would use the statement “isn’t it obvious” again in the future to try to test their ability to avoid going down the garden path.
A great deal of chemistry is involved in the dissolving of an Alka-SeltzerTM table. Point out to students that Alka-SeltzerTM is composed mainly of “Heat treated sodium bicarbonate” (in other words, dry baking soda), some acetylsalicylic acid (ie. Aspirin) and citric acid. The purpose of the citric acid is to react slowly with the baking soda to produce bubbles as soon as the tablets are “plopped” into water and the aspirin provides pain relief. Why baking soda? Studies showed that stomach remedies are thought to be more effective if they produce a satisfying “burp”, which of course happens when the baking soda reacts with the hydrochloric acid in the stomach. When an Alka-SeltzerTM is dissolved an almost-insoluble white residue of aspirin remains. Hence, Alka-SeltzerTM is effectively a very expensive way to take an aspirin!
Heroes and heroines in action movies are frequently seen running down a hallway while a wall of flame from an explosion is chasing them down the hallway, such that they crash through a window just as the flames erupt out the window with explosive force. There is a small problem with such scenes: “flame-front” velocities are in excess of 20 km/s (that is, 72,000 km/hr)! If people could run that fast they and their clothes would burst into flame in the same way that a meteor burns as it enters the earth’s atmosphere.
Reactions in the solid phase, although rare, do occur and can be quite fast. For example, one day an unknown student at Kamloops Secondary took too much colourless sodium iodide and put it back in a bottle he/she thought contained sodium iodide but instead contained colourless lead nitrate. Instantly, the lead and iodide ions reacted to form bright yellow lead iodide! The mistake was noticed by the next student to use the contaminated bottle and the entire bottle had to be discarded. This can be a cautionary story to relate to students as to why chemicals are NEVER returned to a stock bottle and also serves as a lesson to green teachers who thoughtlessly put out an entire stock bottle of a chemical rather than pour out the required amount for the class into a separate beaker with an unmistakable label.
An interesting effect was found in the UBC Chemistry department in the 1960’s: a chemical reaction was discovered whose reaction rate increased hugely then the temperature was decreased. For a while, the entire department became excited about this apparent violation of a commonly-accepted chemical principle. The mystery was resolved when it was found that as the temperature dropped the solvent froze in a loose cage structure and forced the reactive solutes into tiny liquid pockets that now contained reactant concentrations that were hundreds of times greater than those found in the previous solution. The now-concentrated chemicals then reacted at a fast rate because of their massively-increased concentration and the temperature became a secondary and relatively unimportant factor.
Understanding kinetic energy distributions is usually difficult for students, at least when first encountered. A helpful analogy is to consider 1000 full term newborn infants and plot a histogram of number of children (height of rectangle) versus length of child (in 1 cm intervals). This histogram spreads out over a small number of centimeters and there will be many children in each interval. (This is analogous to the appearance of the kinetic energy distribution at low temperatures.) If a histogram is again plotted when the children reach the age of 18 (assuming there are still 1000 individuals), the number of individuals in each height interval will be much less than in the previous graph because the range of heights is much larger – a little humour can be introduced if one of your students is a star player on the school’s basketball team, as I found when one of my students was 6’10” tall! (This is analogous to the kinetic energy distribution at high temperatures.)
The 2nd diagram on page 18 of the Chemistry 12 Workbook is frequently misunderstood by students. A good analogy that some puzzled students seem to understand is to think of a rope tied at one end to a clothesline and at the other end tied to a post from which the clothesline is attached. When the rope on the clothesline is close to the end near the post, the rope hangs far down, almost touching the ground. When the clothesline is pushed out the end of the rope attached to the line will be farther from the post and the middle part lifts up from the ground. If this situation is looked at “upside down”, the same effect is seen in the diagram. As the end of the curve recedes farther from the vertical axis, the height of the curve decreases.
The gain and loss of kinetic energy that accompanies reacting molecules can be made more concrete for some students if you ask them to think of the potential energy diagram on page 20 of the Chemistry 12 Workbook as being a real-but-tiny hill and think of the reactant molecules as being marbles that are being rolled over the hump. This visual analogy lets them clearly see that the marbles (molecules) slow down as they get approach the top of the hill and then gain speed as they roll down the other side, If the reaction is exothermic, they should see that if a marble started with a speed just barely sufficient to get it over the top then by the time it reaches the lowest point on the other side the marble/molecule will be traveling much faster than it was originally.
Students taking Biology 12 are frequently confused if they try to resolve the biology concepts involving energy production when ATP (adenosine triphosphate) is converted to ADP (adenosine diphosphate) and AMP (adenosine monophosphate) and the chemistry concept that the breaking of a bond requires an input of energy. At first, it appears that a phosphate bond is broken in ATP and energy pours out in the same way that breaking an egg releases the egg white and yolk. This is the opposite of what the chemist is saying! However, the ATP reaction is actually a hydrolysis: ATP + H2O ===> ADP + H3PO4 (Strictly speaking, at pH 7 the phosphoric acid units of ATP, ADP and AMP are completely deprotonated.) Since there is both bond breaking (H-OH in water, and the P-O bond linking the terminal phosphate to the second phosphate) and bond making (an O-H bond and a P-OH bond form), the exothermic character of the bond making is in excess of the endothermic character of the bond breaking, so that the overall reaction is exothermic by about 7 kJ/mol .
The kinetic energy distribution at the top of page 18 of the Chemistry 12 Workbook is called a Boltzmann distribution. Ludwig Boltzmann was one of the greatest theoretical physicists of all time. If Ludwig did not know about some point of physics, nobody did. His reputation was enormous but unfortunately he suffered from bouts of depression. Shortly after Einstein published his famous paper on Special Relativity in 1905, Ludwig read the paper and realized that Einstein had just “blown away” a huge chunk of classical physics, including much of Boltzmann’s life work. In 1906, suffering from acute depression possibly brought on by the effect of Einstein’s theory, Boltzmann committed suicide. In an interesting turn of events, Einstein was unable to complete his general theory of relativity until 1916 because of his lack of mathematical background. One of the breakthroughs came when Einstein realized that Boltzmann’s theories were needed to shore up the mathematical underpinnings of general relativity. Boltzmann took his life because he was mistaken about the significance of his work. History now judges Boltzmann very well for his brilliant contributions to physics.
Exercise 39, page 23 of the Chemistry 12 Workbook. A chemist in the early part of the 20th century spent years trying to make diamond out of graphite. He tried different catalysts, different reactants, different temperatures, different pressures and different combinations of all the foregoing conditions. One day, on April Fool’s day, one of his graduate students slipped a small diamond chip inside one of the reaction vessels and intended that the professor should open the vessel, find the diamond and be startled by the graduate student jumping out of his hiding place to cry “April Fools”. Unfortunately, the graduate student was delayed by a late or missed streetcar connection and was not there when the professor opened the vessel, found the diamond and ran across the campus telling anyone and everyone he found that his years of searching were over. The professor’s reaction is not recorded when the sheepish graduate student finally arrived and confessed to the prank. NASA has suggested that on long space flights food will have to be synthesized aboard the spacecraft. One suggestion that NASA has put forth is the following set of reactions.
Old chemists never die, they just fail to react.
Teacher to pesky student: Warning; I have a very low activation energy!
[Chem 13 News, April 2001, p.7]
Unit II: Equilibrium
An effective way to illustrate how temperature affects an equilibrium reaction is to bring two volunteers to the front of the room, place them facing each other about 1.5 m apart such that one is offset from the other to allow them to both take a couple of steps forward to the opposite side without colliding. One student is on the reactant side and the other is on the product side of the room. The teacher points out that s/he represents heat energy and stands on the right side, behind the “product” student. The rules of the demonstration:
- students move to the other side (if possible) each time the teacher claps her/his hands, and when they move they again face each other upon reaching the other side.
- whichever student is on the reactant side automatically moves to the product side when a hand claps.
- whichever student is on the product side can only move to the other side if given a gentle push on the shoulders (corresponding to heat being required).
Start the process, clapping and simultaneously pushing forward the student on the product side. After a couple of clap and push intervals, clap and then DON’T push the student. The “reactant” student usually hesitates and then steps forward, leaving both students on the same side. Point out that when the temperature is decreased the reaction shifts to the product side of an exothermic reaction.
'Smelling salts' is the common name for ammonium carbonate, which is actually a mixture of ammonium hydrogen carbonate (NH4HCO3) and ammonium carbamate (NH4CO2NH2). Your great grandfather probably carried a small container of smelling salts in his vest pocket to help revive your great grandmother after she had one of her fainting spells. (Victorian ladies were considered to be frail creatures that 'swooned' whenever anything startled or frightened them.) When the smelling salts were placed under great grandma’s nose, some of the white powder quickly decomposed in an equilibrium reaction into three gases: ammonia (NH3), carbon dioxide (CO2) and water vapor (H2O). The strong smell of ammonia was quite sufficient to revive a person who had fainted.
A nice analogy to help see how Le Chatelier’s Principle operates is the following. Pretend you have a fish tank with a divider down the center. Further, pretend the divider has a small hole at the bottom, so that water can pass from one side of the tank to the other by passing through the hole. Assume that a bucket of water has been added to the left (“reactants”) side. Students can easily see that some of the added water will flow to the other side. If one used a bucket to remove water from one side, water would flow from the other side to make up the loss of water.
Grecian Formula for Men consists of, among other ingredients, a dilute solution of lead(II) acetate. The lead(II) ion combines with the sulphide linkages in hair to make lead (II) sulphide (the grey mineral “galena”) and darkens the hair shafts.
Although gold is almost completely insoluble in water, nevertheless tiny amounts of gold will dissolve. Prospectors sampling some “played out” gold fields in the Yukon found unexpectedly large amounts of gold in areas that were more-or-less continually moist. The gold had an unusual appearance, and when examined under a microscope it was found that the gold consisted of masses of tiny elongated tubes. Further investigation showed that some bacteria isolate and concentrate the minute amounts of gold in the ground waters and secrete the gold as protective coats. Bacteria were essentially re-growing the gold fields! In a similar vein, geologists in the 1960’s found that the roots of evergreen trees absorb and concentrate the minute amounts of minerals that dissolve in ground waters, so that the trees secrete tiny droplets of gold, for instance, in the growing tips of their needle clusters. By sampling and analyzing the minerals in the tips of tree branches, geologists are able to tell what minerals are buried deep underground.
It is possible to use language that cause people to worry about situations that do not pose any threat. For example, the Kamloops area contains mercury, including a played-out mercury mine. If you see someone drink water from Kamloops Lake, you could tell him that “that water you are drinking is saturated with mercury!”. In fact, the water is virtually free of mercury because the area also contains small amounts of sulphide ions. Since the Ksp for HgS is 6.4 x 10-53, then the [Hg2+] in the “saturated” mercury solution is 8 x 10-27 M, which mean one would have to drink an average of about 200 L of water to ingest one mercury ion. “Saturated” doesn’t mean “concentrated”.
Gas solubility decreases with increasing temperature (greater temperatures favour an equilibrium shift to a state with higher entropy), so that the dissolved state shifts to the gaseous state. Examples of this: open bottles of soda pop go “flat” quicker at room temperature than in a refrigerator; the South Seas may have beautiful life forms but they are almost marine “deserts” because the lower oxygen concentrations at the higher water temperatures preclude an abundance of life – on the other hand, cold waters such as those in the Japan Current contain high oxygen concentrations and a massive abundance of life, as witnessed by divers off the breakwater in Victoria BC. The marine forests off the Victoria breakwater are lush and almost impenetrable with kelp, fish and other marine life.
Unit IV: Acids, Bases and Salts
In his Ph.D. thesis in 1884, Arrhenius proposed the theory of what we now call ionic solutes, including positive and negative ions, and the interaction of the solvent with salts, acids and bases. Because some of the most prominent men in science at the time did not believe in the existence of ions, let alone that they should interact, and because some of his proposals were poorly supported by vague evidence, the committee judging his oral examination and thesis gave him the lowest possible passing mark (because they could not actually disprove his work), knowing that Arrhenius was now doomed by being unable to get an academic position in his homeland, Sweden. It was only when a famous German physical chemist, Wilhelm Ostwald offered Arrhenius a position because of the brilliance of his thesis work that the Swedish relented and admitted him to the ranks of Swedish scientists. He went on to become one of Sweden’s most influential scientists and in 1903 was awarded the Nobel Prize in Chemistry for his work on ionic solutes.. In an ironic twist of fate, Albert Einstein was turned down for a Nobel Prize in physics several times because many lesser scientists thought his theory of relativity was flawed. The committee member who kept vetoing Einstein’s award? Arrhenius! (Einstein was eventually given the Nobel prize for his less controversial work on the photoelectric effect.) [J.Chem.Ed. Vol.78, No.8, p. 1066 and J.Chem.Ed. Vol. 71, No.5, p. 393]
Although we can’t change what is written in textbooks (and curriculum guides!), Lowry made it very clear that he did not claim any credit for Brønsted’s concepts and hence we should simply refer to the Brønsted Theory, not the Brønsted-Lowry Theory. [J.Chem.Ed, volume 75, No. 4, p. 410]
Perchloric acid, HClO4, is so strong that if pure HClO4 is mixed with pure H2SO4, a strong acid, the following reaction occurs: HClO4 + H2SO4 ===>ClO-4 + H3SO+4
The Brønsted-Lowry theory of acids and bases is sexist as usually presented. The usual theory starts by defining an acid as a proton donor and a base as a proton acceptor. Since the alchemical symbol for an acid is the same as the biological symbol for “male” (a circle with an arrow slanting to the right), and since most alchemists were male, as were both Brønsted and Lowry, a certain sexual bias naturally crept into the theory. Everything is defined from the viewpoint of an acid (H+, the proton). An obvious acid-base equation which restores the balance is:
Look at the equation for a moment … water is acting as a hydroxide donor and BeOH+ is acting as a hydroxide acceptor. Aha! This equation completes the Brønsted-Lowry definitions. In this case, “base” takes centre stage (the female symbol in biology equates to the alchemical symbol for “base”) because the base is now not just a meek proton acceptor, it is a proud hydroxide donor and the acids are relegated to being mere hydroxide acceptors.
The latest evidence shows that when the hydronium ion forms in water the stable species is not H3O+, but rather is H3O+4 (each H in H3O+ is strongly hydrogen-bonded to the oxygen of an additional water). Higher hydration numbers are also seen on a more transient basis. Make sure students know that they should still refer to H3O+ when discussing acid solutions since the course has not caught up with their new knowledge ;-)
When introducing logarithms, an approach that students find very useful is to have them consider how a junior math whiz (whose mom has an advanced degree in mathematics and whose dad has an advanced degree in chemistry) answers the following question in class: “what is the answer when you multiply 2 times 3?”. Little Suzy proudly marches up to the front and writes on the blackboard: 100.3010 x 100.4771 = 10(0.3010 + 0.4771) = 100.77881 = 6! (Memorize the fact that log(2) = 0.3010 and log (3) = 0.4771 ahead of time.) Since students already understand the rules for multiplying exponential numbers, they will nod in agreement while being puzzled by “where did you get those exponential numbers?” Ask them to locate the 10X key on their calculator and enter: 0.3010, 2nd, 10X (this is the usual key sequence). They should get “1.99986…”. Caution them not to clear the display. Now point out that (usually) the 10X key is on the same button as the log key (which is usually written below it on the same key). Tell them to press the log key; they will get “0.301” back again. Aha! Now they know where Suzy got 0.3010. It is the log of 2. Have them check Suzy’s answer by calculating the log of 6. Now stress the fact that they know that 1 = 100 and that 10 = 101, but how do they deal with 5 = 10X ? Logs!!! So taking the logarithm of a number is just a fancy term for finding the power of 10 needed to express the number. The fact that to multiply (or divide) two numbers you just add (or subtract) their logarithms just follows from the well-known fact that you add (or subtract) exponentials when multiplying (or dividing) numbers. Also, 2 log (X) = log (X) + log (X) = log (X2) follows directly from the rules for dealing with exponentials. This overall approach takes about 5-10 minutes to introduce logarithms and pays off in much better student comprehension.
When introducing the concept of pH, and universal indicator solutions, have your students deliberately place a drop of universal indicator solution on a blank page of their lab text. The indicator usually turns red (check this ahead of time!), indicating a pH of about 4. Point out that a large part of our heritage and knowledge is printed in books containing acidic paper. Such paper slowly decomposes and crumbles after a few decades. Many scientists are investigating ways to neutralize the acid in the paper in order to prevent the loss of all this knowledge in the future. The crumbling of newspaper shows this acidic effect very well. A piece of newspaper left outside in the sun will turn yellow after a few days and will eventually get brittle. The cellulose in the paper is decomposed into simple sugars by the acids in the paper and the sugars start to “caramelize” (turn yellow) as the sun’s energy starts decompose the sugars.
Many types of flower petals contain indicator dyes which change colour depending on the pH. Make a “tea” from such flower petals and check the colours when either vinegar or baking soda is added. One gardener in Vancouver with a unique sense of humour planted a row of hydrangea bushes and made the soil acidic on the left-most set of bushes by adding aluminum sulphate. He made the soil on the right-most set of bushes basic by adding powdered limestone (calcium carbonate). When the bushes flowered, the plants in acidic soil had pink flowers and the plants in the basic soil had blue flowers. The next year, he reversed the acidity on the left and right sides, thereby creating different coloured flowers from those of the previous year. Puzzled neighbours thought he had dug up the bushes and rearranged them.
Max Perutz and John Kendrew won the Nobel Prize in Chemistry in 1962 for their work in determining the exact structures of myoglobin and haemoglobin. In 1968, Perutz gave a lecture at UBC outlining his work. By then he was an old man but still bounced around with the energy of a twenty year old, so great was his love of his work, and what he described was simply incredible. The haemoglobin molecule contains about 10,000 atoms and has an Fe2+ ion held in the middle of a flat assembly of other atoms. When an oxygen atom attaches itself to the Fe2+ ion, the Fe2+ changes to Fe3+, which is smaller in size. This smaller ion pulls the surrounding atoms attached to the iron more strongly, distorting the flat shape and causing another part of the haemoglobin molecule to close over and trap the oxygen molecule, like a clamshell closing. When the haemoglobin molecule is transported to a region where cells are producing CO2, a CO2 molecule attaches itself to the Fe3+ ion on the outside of the now-closed haemoglobin molecule. The CO2 changes the Fe3+ back to Fe2+, opening up and flattening out the molecule, and ejecting the O2 molecule so as to be available for the cell’s respiration needs. The haemoglobin molecule is then recycled back to capillaries lining the lungs, where an O2 attaches itself, changes the Fe2+ to Fe3+, distorting the flat arrangement, closing the molecule like a clamshell and ejecting the CO2, where it is transported out of the capillaries into the lung’s airspace and exhaled.
High carbon dioxide concentration in the bloodstream can lead to interesting hallucinations. For example:
- Ballet dancers who do intense physical exertion without breathing properly get light-headed and start to hallucinate.
- During the Crusades, a fanatic sect called the Dervishes would dance in a whirling, rotating manner as part of a religious rite (which also happened to involve the consumption of hashish) until they were “higher than a kite”. At the height of their “elevated state”, wearing no armor and armed only with a sword and knife they attacked heavily armed crusading knights in full armor with devastating consequences, slaughtering large numbers of knights.
- The so-called “near-death experience” is largely an effect of carbon dioxide buildup in a brain that is going anoxic. If a person stops breathing, the brain is still active and even if the eyelids are shut light can still penetrate the lids and be perceived. It is well known that a buildup of CO2 preferentially shuts down the rods on the periphery of the retina, leading to a “tunnel effect” (light is perceived coming from directly ahead but not from the sides). In addition, the hallucination effect of CO2 buildup distorts voices heard around the room, usually being perceived as a friendly, beckoning voice from a distance.
Unit V: Electrochemistry
Students sometimes have a problem remembering that on the chart of Standard Reduction Potentials, the reduction reaction must be above the oxidation reaction in order to be spontaneous, and that the reduction reaction “goes forward” (as written on the chart) while the oxidation reaction “goes backward” (as written on the chart). A nice analogy that nails the concepts firmly in student’s minds is to point out that if a student is asked to sketch a side view of a swimming pool with a low diving board at one end, they will almost always draw the pool and board with the diving board on the left end, like this:
Now to complete the analogy: a person will run forward (to the right) on the board, dive down and swim back (to the left) to get out of the pool. This set of 3 directions mimics the “reduction goes forward”, move down on the table to find the oxidation and then “oxidation goes backwards”.
When the British navy started using copper sheathing on their wooden-hulled boats, they quickly found a serious problem: the copper sheets quickly corroded, exposing the underlying wood to toredo worms. Hence, the Navy commissioned Sir Humphrey Davy to investigate ways to prevent the corrosion. In 1824 he discovered the principal of “cathodic protection” and found that attaching strips of zinc, tin or iron protected the copper sheets. However, iron was not practical since it rusted quickly and tin was expensive, so that zinc quickly became the metal of choice. As a result, zinc went from being an almost worthless, dull and unattractive weak metal to a valuable commodity almost overnight. Many mines in the interior of B.C. were developed solely to provide zinc for the growing need for zinc as a cathodic protectant
What we call “sold rock” isn’t. Geologists have found that the most solid-appearing rocks have huge numbers of tiny cracks that allow air and water to penetrate for several thousand feet below the surface. This upper portion of the earth’s crust is therefore called the oxidation zone because any minerals that are attacked by water and oxygen are converted to other minerals. Only below this oxidation zone can some minerals be found. Another interesting outcome of this interconnected system of air- and water-filled cracks is the discovery that bacteria have been found up to several miles below the surface. Many biologists now believe that the total mass of bacteria living deep below the surface now outweighs the total mass of all other life on earth.
For a few years, aluminum wiring was used in constructing new homes. Then, a series of house fires were caused by this wiring overheating and causing the aluminum and surrounding wood to ignite. The problem was that aluminum immediately forms an oxide coating which happens to be a poor conductor. In addition, when the aluminum wires were attached to copper screws and other connecting devices, corrosion caused the aluminum to oxidize to an even greater extent. Because of this increased resistance, the aluminum became hot as electricity passed through the resistive layer and a fire frequently started.
Today’s students show virtually no fillings when they open their mouth to laugh. However, their parents usually have a mouthful of fillings and probably have experienced an electrochemical reaction in their mouths. If they accidentally chew on a piece of aluminum foil it causes an electrical current to flow through a filling to the underlying nerve and creates a painful electrical shock.
Normal paper contains starch, which reacts with iodine to produce the blue-black starch-iodine complex, but paper currency is manufactured on special starch-free paper and does not react with iodine. Banks and many stores now use pens containing iodine solution to quickly make a mark on paper money. Genuine bills leave a yellow mark and counterfeit bills leave a black mark. [Chem 13 News, May 2002, p. 3]
Vitamin C is added to apple juice not just to increase the nutritional value of the juice. Vitamin C prevents the juice from oxidizing and turning brown in the same way that a ripe apple slowly turns brown from aerial oxidation after several bites are taken out of the apple and the apple is set aside for a minute or two. Interestingly, apple juice in Russia IS brown because it does not contain vitamin C.
People who drink over 50 mL of methanol (methyl hydrate) will die if not treated almost immediately: the methanol is oxidized in their livers to formaldehyde. Physicians treat methanol poisoning by orally administering large quantities of ethanol (drinking alcohol). This treatment occupies the liver function as it oxidizes the ethanol preferentially, so that the methanol is simply excreted in the urine in an unchanged form. When the urine output of the catheterized patient shows the methanol output is virtually zero, the patient is allowed to sober up. The treatment takes more than one day.
Regular dry cells and alkaline batteries have the following voltage versus time behaviour:
whereas the cells in pacemakers have the following behaviour:
Pacemaker batteries usually last 5-10 years and are usually lithium iodine batteries. Consider why the lower type of voltage-vs-time behaviour is essential: the top type will produce a slower and slower “flub-dub” heart rhythm. Of course if the pacemaker battery is not replaced within a reasonable safety margin there is a danger of “flub-dub, flub-dub, flub…” (no dub!)
As a final project in Chem 12, why not have students silver plate a “grad penny”? Go to a bank and get a roll of new pennies. Make up a 0.1 M solution of silver nitrate (say 500 mL) and add a 1 M solution of sodium iodide. Initially, a yellow precipitate of silver iodide is formed (do this part in subdued lighting since silver iodide is photo-sensitive), but continued addition of sodium iodide eventually causes the precipitate to dissolve again as the Agl-2 ion is formed. The final solution is pale yellow. (If nervous about using all this silver nitrate, try the procedure by initially making up 25 mL of 0.1 M AgNO3.) Since the [Ag+] is very low in the NaAgI2 solution, the reduction potential for Ag+ is lowered sufficiently so as to be lower than the reduction potential for Cu2+/Cu and hence will not spontaneously deposit silver metal on a copper surface. Next, make up a small amount of 6 M nitric acid, 100 mL will do, to clean the surface of the copper. Get a 6 V DC power supply from your Physics teacher and a pair of electrical connectors with alligator clips on the end. You will also need an inert electrode such as a carbon rod and a porous ceramic cup. Place the ceramic cup inside a clean 250 mL beaker and place the carbon rod inside the ceramic cup. Pour enough NaAgI2 solution into both the beaker and ceramic cup to bring the liquid level to about 4-5 cm in height and attach one alligator clip to the carbon rod. Attach the other alligator clip to the edge of the penny, dip the penny in the nitric acid for 2-3 s, remove the penny from the acid solution and run the penny under tap water to wash off the nitric acid. Place the penny and alligator clip in the NaAgI2 solution in such a way that the penny is suspended in the solution without touching the beaker or ceramic cup (a test tube clamp on a ring stand may help). Connect the electrical leads to the power supply in such a way that alligator clip attached to the penny is the cathode and the carbon rod is the anode. Turn on the power supply and let the current flow for about 3-5 minutes. Remove the penny from the solution, wash it under a gentle flow of water and place it on a folder paper towel. Remove the alligator clip. The penny should have an adherent coating of dull grey silver on it, with no unplated sections evident except where the alligator clip contacted the edge. Make a buffing pad out of another piece of folded paper towel and use powdered chalk (powdered calcium carbonate) to buff the penny to a bright silvery shine.
During an Oxford oral examination in 1890, the examiner asked an aspiring student if he knew what electricity was. “Oh sir, I’m sure I’ve learnt what it is. I’m sure I did know – but I’ve forgotten,” answered the nervous candidate. “How very unfortunate,” retorted the examiner, unimpressed, “Only two persons have ever known what electricity is, the Author of the Universe and yourself. Now one of them has forgotten.” [New Scientist, December 25, 1993, p. 41]
William Gladstone, then Chancellor of the Exchequer, was invited to a demonstration of Michael Faraday’s equipment for generating the latest scientific wonder – electricity. Faraday set up the experiment and ran it, while Gladstone looked coolly on. When the show had run its course, Gladstone stood silent for a moment, and then said to Faraday” It is very interesting Mr. Faraday, but what practical use has it?” “One day, sir, you may tax it,” replied Faraday. [New Scientist, December 25, 1993, p. 41]