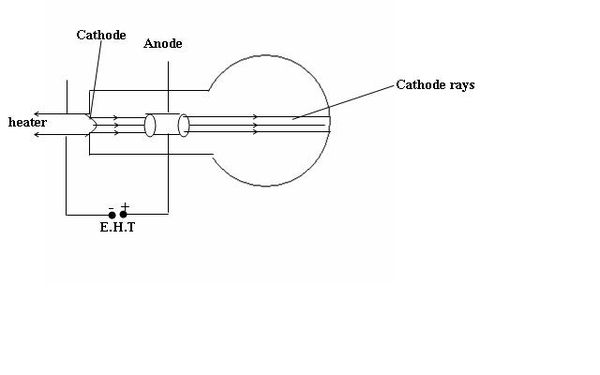
CATHODE RAYS
Definition. Cathode rays are highly energetic electrons moving from the cathode to the anode. They are produced in a cathode ray tube. Production of cathode rays
The electrons are produced at the cathode by thermionic emission and are accelerated towards the screen by the anode which is connected to the terminal of the extra high tension battery. The thermionic emission is the process whereby metal surfaces emit electrons when heated. The tube is evacuated to avoid electrons interacting with any particle before they reach the screen. When the cathode rays hit the florescent screen, the screen glows. This shows that electrons posses momentum and therefore have mass.
""'Properties of cathode rays""' i. They are negatively charged ii. They travel in a straight line iii. They are deflected by both magnetic and electric fields (this proves that they carry a charge) iv. They cause fluorescence in certain materials. v. When cathode rays are stopped by heavy metals, x-rays are emitted. vi. They are electrons moving with high speeds.
Verification that electrons travel in a straight line
If an opaque object (Maltese cross) is placed in the path of the cathode rays, a sharp shadow of the Maltese cross is cast on the screen.
Shadow of the Maltese cross cast on the florescent screen
CATHODE RAY OSCILLOSCOPE (C.R.O)
Uses of the parts
Evacuated Glass tube:- the glass tube evacuated to prevent scattering of the electron beam when electrons collide with air molecules.
Indirectly heated Cathode, C :-Emits electrons by thermionic emission.
The grid, G:- it consists of a hollow metal cylinder with a small hole at the end. It is held at variable negative potential relative to the cathode by means of the potential divider R1, The grid serves two purposes namely: (i) as a brightness control
(ii) it refocuses the electron beam so that the beam emerges from the hole as a narrow beam.
Anodes A1 and A2:- These are held at a positive potential relative to the cathode. The anode accelerates the electron beam along the tube and also focuses the electron beam by means of the potential divider R2.
X-plates, X1 and X2:-these are vertical plates but they deflect the beam horizontally when a p.d is applied across them.
Y-Plates, Y1 and Y2:- These are horizontal pates but deflect the beam vertically when a p.d is applied across them.
Fluorescent Screen:- This is coated with fluorescent material such as Zns. It enters light when struck by electron beam.
Graphite Coating:- Enables light to be seen only on the screen as the graphite coating absorbs the electron’s kinetic energy.
Power supply:-This is a smoothened rectified a.c, fed through a chain of resistors.
Operation of the CRO
Suppose the X- plates were shunted and a d.c voltage was applied to the Y- plates. The electron spot would be deflected vertically.
If the X- plates are shunted and an a.c is applied to Y-plates, the electron beam is drawn into a vertical line.
To observe the waveform of the a.c signal applied to the Y-plates, a special voltage called time base connected to the X- plates. The time base has a saw-tooth waveform and is generated by a special in the CRO. The saw tooth voltage which sweeps the electron beam from left to right at a constant speed.
The time taken for p.d to fall from A to B known as the fly back time, is extremely small compared to the time taken to rise from O to A. Hence the time taken by return of the electron beam to the original position at the other end of the screen is small. When no signal is applied to the Y- plates, the voltage V, causes the electron beam to sweep horizontally to and fro on the screen as shown.
To observe the wave form of the applied voltage to Y-plates, the frequency of the time base is synchronised with the frequency of the signal applied to Y-plates. For an a.c signal applied across the Y- plates and time base on the X-plates, one observes the waveform shown below;
Uses of a CRO a) Displaying of waveforms: the amplitude and frequency of the wave can be obtained. b) Measurement of Voltage: An unknown voltage is applied across the Y- plates. If the time base is switched off, a vertical line is obtained on the screen. This can be centred and its length measured. This is proportional to twice to the amplitude or peak voltage, V0. c) Comparison of frequencies of two waveforms: suppose two waveforms of frequency f1 and frequency f2 appear on the screen of the CRO having two Y-inputs or are displayed at a time on the CRO with a single Y-input. If x1 and x2 are distances occupied by one cycle for two waveform, then the ratio , where T1 and T2 are the periodic times of the two waves respectively. d) Measurement of phase difference using a double beam CRO: the two waveforms to be compared have the same frequency. Suppose they are displayed simultaneously by applying them to the two Y-input. Comparison o CRO with a moving coil Voltmeter. a) The CRO has very high impedence. It gives accurate voltages than a moving coil voltmeter. b) A CRO can measure both d.c and a.c voltage. A moving coil voltmeter measures only D.C voltages unless a rectifier is used. The CRO gives a peak to peak values of a.c. c) A CRO has negligible inertia as compared to a moving coil voltmeter. The CRO respond almost instantaneously. d) CRO doesn’t give direct voltage readings.
Question A CRO has its Y- sensitivity set to 10Vcm-1. A sinusoidal input is suitably applied to give a steady trace with the time base set so that the electron beam takes 0.01s so traverse the screen. If the trace seen has a total peak to peak height of 4cm and contains two complete cycles, what is the r.m.s voltage and frequency of the input? (14.1V, 200Hz)
Photoelectric effect When some metals held at a negative potential are illuminated by electromagnetic radiations, electrons are emitted. This process is called photoelectric emission. Demonstration of photoelectric effect
When light falls on a metal cathode, a galvanometer shows a deflection, indicating flow of current. However when the plates are covered, more current flows. Energy of the incident light is absorbed by the electrons and instantly an electron jumps out. Such ejected electrons are called photoelectrons.
Experimental observations on photoelectric effect.
1. There is negligible time delay between irradiation of metal surface and emission of electrons by the surface.
2. The photocurrent( number of photoelectrons per second) is proportional to the intensity of the incident radiation.
3. The maximum kinetic energy of photo electrons increases linearly with the frequency of the incident radiation but is independent of the intensity of the radiation.
4. For each metal surface, there is a minimum frequency, f0 of the incident radiation below which no electrons are emitted however high is the intensity. This frequency is called Threshold frequency of the metal surface.
X – Rays X- rays are short wavelength electromagnetic waves which are produced when cathode rays are stopped by heavy metals. Production of X – rays
Mode of operation
A low voltage is applied across the filament and heats the filament. Electrons are emitted by the filament by thermionic emission. The concave focussing cathode focuses the electrons from the filament onto the target. A very high alternating voltage is applied between the filament and the anode. During the half cycles when the anode is at a positive potential relative to the cathode, electrons are accelerated across the tube. No electrons flow to the anode when the anode is at a negative potential relative to the cathode.
When the cathode rays (electrons) strike the target, 99% of the kinetic energy of electrons is dissipated into heat while 1% is turned into X-rays. The heat generated at the target is cooled by means of the copper cooling fins mounted on the copper anode. Heat is conducted from the target away from the tube by conduction and radiation. The electron current, I in an X-ray tube in operation is given by I = ne, where n is the number of electrons per second and e is the electronic charge.
Intensity of X-rays (Quantity) The intensity of X- rays in an X – ray tube is proportional to the number of electrons colliding with the target. The number of electrons produced at the cathode depend on the filament supply. The greater the heating current, the greater the number of electrons produced and hence more x- rays are produced. Therefore the intensity of X- rays is controlled by the filament current. Penetration of X – rays ( quality) Penetration power of X-rays depends on the kinetic energy of the electrons striking the target. The higher the accelerating voltage, the faster the electrons produced. Faster electrons posses higher kinetic energy and shorter wavelength x-rays of greater penetration power are produced. Hence penetrating power of X-rays is determined by the accelerating Voltage across the tube. Hard and soft X- rays Hard x-rays have a high penetrating power. This because they have very short wavelengths. They are produced when a high p.d is applied across the tube. Soft X-rays are produced by electrons moving at relatively lower velocities than those produced by hard x –rays. They have less energy, longer wavelengths, hence less penetration power compared to hard x-rays. Hard x-rays can penetrate flesh but are absorbed by bones. Soft x-rays are used to show malignant growths since they only penetrate soft flesh. They are absorbed by such growths.
Properties of X –rays
o They travel in a straight line at a speed of light
o They are not deflected by both magnetic or electric fields. This indicate that they carry no charge.
o They penetrate all matter to some extent. Penetration is least in materials with high density and atomic number e.g. lead.
o They ionise gases through which they pass.
o They affect photographic plates just like light does.
o They cause fluorescence in some materials.
o They cause photoelectric effect when they are illuminated on certain metal surfaces.
o They are diffracted by crystals leading to an interference pattern.
Uses of X-rays
1. Structural analysis, stresses, fractures in solids, castings and welded joints can be analysed by examining X-ray photograph.
2. Crystallography; Orientation and identification of minerals by analysis of diffraction patterns using Bragg’s law.
3. Medical uses;
(i) Analytical uses. These include location of fractures, cancer and tumour/defective tissue absorbs x-rays differently from normal tissue. (ii) Therapeutics use for destroying cancerous cells and tumours.
1. detection of fire arms at international airports.
Thermionic Diode
Structure It consists of an anode usually in form of a nickel cylinder which surrounds the cathode in an evacuated glass bulbs. In the indirectly heated cathode type, the cathode is a nickel tube with a tungsten filament (or heater) inside it. The heater is insulated electrically from the cathode by packing alumina inside the nickel tube. The outside of the tube is coated with a mixture of Barium and Strontium oxides. The mixture has a low work function(about 1.8eV) and emits electrons at relatively low temperatures (about 1100K)
Symbol of a diode
Thermionic diode Characteristics
Keeping the filament current If constant, the p.d Va between the cathode and the anode is varied. The corresponding anode current Ia is measured. A graph of Ia against Va constitutes the anode – current anode voltage characteristics. By setting the filament current to other constant values, the corresponding Ia-Va characteristics can be obtained. These features can be shown below
For Va = 0 ,electrons are emitted by the cathode with a range of speeds. A few of the electrons are emitted with sufficient kinetic energy to be able to reach the anode. This leads to a small current. If the anode is made negative relative to the cathode, a reverse current exists for negative potentials up to about 0.5V and then decreases to zero. Region AB: Here Va is small. Only those electrons emitted with high speeds will be able to reach the anode. The majority of the electrons are emitted with low kinetic energies and are repelled back towards the cathode. The electron distribution around the cathode constitutes a negative space charge. The current Ia is small. Region BC: as Va increases the attraction of the space charge by the anode increases. This results in a larger anode current. This region is called space charge limited region. Region CD: the anode voltage Va is so large that all the electrons emitted per second by the cathode reach the anode. The space charge is overcome. A constant current, called saturation current flows. Region CD is also called the temperature limited region because when the temperature of the cathode increases, more electrons are emitted per second by the cathode. A higher saturation current therefore flows. Applications of the thermionic diode (a) Half- wave rectification Suppose a thermionic diode is connected in series with a source of alternating voltageVi and a load RL
During the half cycles when A is positive relative to C the diode conducts and a p.d VR appears across the load RL. During the half cycles when A is at a negative potential relative to C, the diode does not conduct and no p.d appears across RL. The a.c is half-wave rectified. The input and output voltage wave forms are compared in the diagram below.
(b) Full wave rectification
(i) Using two diodes
When P is at negative potential relative to Q, diode D1 conducts whereas D2 doesn’t. When P is at a positive potential relative to Q, diode D2 conducts whereas D1 does not. Current flows in the same direction through the load RL during both positive and negative cycles of the input voltage Vi.
VD1 and VD2 is output pd due to conduction of diode D1 and D2 respectively.
VR is the output voltage across load RL.
(ii) Using four diodes
The following rectifier symbols will be used.
During the half cycles when A is at positive potential relative to B, diodes D2 and D3 are forward biased hence they conduct and current flows through the resistor R in the direction P to Q. Diodes D1 and D4 are reverse biased and they do not conduct. During the half cycles when B is at positive potential relative to A, diodes D1 and D4 are forward biased and they conduct. Currents flows through resistor R in the direction P to Q. Diodes D2 and D3 are reverse biased and don’t conduct. The voltage cross R will have the form:
The output voltage can be smoothened by using filter circuits of the form shown below:
The back emf induced in the inductor by the fluctuating voltage opposes the voltage fluctuations. The capacitor acts as a reservoir to steady the remaining voltage fluctuations. The voltage across the resistor R has the form shown:
At points such as A, the p.d across the load has just reached its maximum value. If the capacitor was not present, the p.d would start to fall to zero along the broken curve. However, as soon as the p.d across the load starts to fall, it becomes less than that across the capacitor and the capacitor starts to discharge through the load.
Nuclear physics
The nuclei of atoms contain protons and neutrons. The collection of protons and neutrons together is called the nucleon.
A species of atoms with a specified number of protons and neutrons is called a nuclide. There are forces which bind the nucleons together. In some nuclides, the forces make the nucleons stay together permanently; however in some, the energy forces binding the nucleus affect some to the nucleons, this happens when the ration of neutrons to protons is big. When ratio is big, the nucleus releases excess energy to become stable.
The number of protons in the nucleus is called the atomic number while the number of protons and neutrons is the mass number.
An atom X, with atomic number Z and mass number A can be symbolised by
A = Z+N, where N = number of neutrons
RADIOACTIVE DECAY
This is the spontaneous disintegration of unstable nuclei emitting alpha, α, beta, β and gamma, γ radiation
Alpha particles
An α-particle is a Helium atom that has two protons and two neutrons. When a nuclei decays by release of an α particles, it loses two protons and two neutrons i.e. mass number decreases by 4 and atomic number by 2.
Alpha particle symbol is
Properties of α particles They cause fluorescent in some materials They blacken photographic plates They readily ionise gases They are easily absorbed by matter. The penetration of matter by α particles is unique in that the α particles can not be detected beyond their range. They are defected by electric and magnetic fields to a lens extent than particles. This means that they are heavier than particles. In both magnetic and electric fields they are deflected in a direction opposite to that of the particles. This indicates that they are positively charged. They are emitted with speeds of the order They are helium nuclei with mass 4U and charge +2e
Beta particles
These are electrons, the mass of the electron is much smaller than that of the proton When an element decays by emitting a particle it loses an electron. Hence the mass number remains the same but the atomic number increase by one. A neutrons is thought to consist of a proton and an electron. When a nucleus disintegration, a neutrons breaks down into an electron ( particle) which is emitted and a proton which increases the atomic number. Properties of particles They have a much smaller fluorescent effect than patties They blacken photographic pates They ionise gases ion readily than particles They penetrate power more easily than α particles but are absorb completely by about 1mmof Aluminium, or a few metres path of air. They don’t have a definite range like α particles owing to successive deflection cause by collision with the atom of the absorber. They are deflected by electric and magnetic fields much more than particles because they are lighter. They are fast moving electrons. They move faster than cathode rays Gamma rays They are electromagnetic radiation with very short ware lengths. These are found to occupy a band the X-ray which are thought to have the shortest ware length known. The main difference between δ- rays and X-rays is that δ -rays originate from energy changes in the nucleus in the atom while X rays originate from energy changes associated with electron structure of the atom. Emission of δ rays has no effect on the mass of the nucleus. Emission of δ- rays is usually accompanied by α or emission e.g.
Properties of gamma rays
Affect photographic plates
They are not deflected by magnetic and electric fields. This implies that they carry no charge
They travel in a vacuum with the speed of light
They are diffracted by light or X rays
Ware length of rays shorter than those of X rays
They cause photoelectric effect i.e. they eject electrons when they fall on certain etals
They have a greater penetrating power than particles i.e. are absorbed by thick lead.
The Decay law
The rate of disintegration of a given sample at any time is directly proportional to the number of nuclide N, present at that time, t.
Mathematically
The negative sign indicates that N decreases as t increases
Decay constant, , is defined as the fraction of the radioactive nuclei which decays per second. The solution of the above equation is A graph of N against t is called the decay curve
Half life The half life of a radioactive source is the time taken for half the number of radioactive nuclei present in the source to disintegrate. Consider the decay curve of a radioactive source
Carbon dating
The unstable isotope 14C produced during nuclear reactions in the atmosphere as a result of cosmic ray bombardment give a small portion of 14C in CO2 in the atmosphere.
Plants take in CO2 for photosynthesis. When a plant dies it stops taking in CO2 and its 14Cdecays to 14N by particle emission.
By measuring the activity of 14C in the remains, the time when the plant died can be estimated.
Radio isotopes
Radioisotopes are nuclides which are unstable and undergo radioactive decay emitting particles or γ- rays during return to a stable form. 238U, 226Ra and 230Th are examples of natural radioactive.
A greater number of radio isotopes are produced artificially by bombarding stable nucleus with high energetic particles such as protons, α-particles, deuterons and neutrons.
Artificial radioisotopes behave the same way as the natural radioactive materials in that each will emit its characteristic particle or radiation and each has a characteristic half-life.
Examples
1. By bombarding
2. Bombarding of boron with particles to get which decays by emission or particles.
then with half life 5730 years. 3. Neutrons are ideal for bombardment of stable nuclei to produce radioisotope because they carry no charge and are therefore not deflected by either atomic electrons or nuclear charge. They will penetrate the nucleus even when their energies are comparatively low. Some uses of radioisotope 1. Biological uses i. Radiotherapy Radio cobalt decays with emission of particles together with very high energy γ-rays. The γ -rays have greater energy than is available with standard X- rays machines when properly shielded, the γ -rays are employed in the treatment of cancer. The iodine isotope (half life 8 days) decays by γ -ray emission. This is injected into the blood stream of a patient having cancer of the thyroid and the γ -rays given off are concentrated right where they are needed. The speed with which the iodine isotope becomes concentrated in the thyroid provides a measure of the thyroid function. ii. Tracers Small qualities of low activity radioisotope are administered by injection into patients and their passage through the body and absorption by diseased tissue studied.
The radioisotope 59 Fe is administered into a patient’s blood stream. Measurements of the radioactivity of a plasma sample will indicate the amount of dilution and hence the total number of red blood cells can be determined if some of the patients own red cells are labelled with 59Fe or 51 Cr and returned into the blood stream.
In agriculture, traces have been used to study how fertilizers, hormones, weed killers and pesticides perform their functions. E.g. the radioisotope has been used to provide in formation about the best type of phosphate fertilizer to supply to particular crops and soil.
iii. Mutants
Radioisotopes have been used to induce plant mutations. This has led to improved seed varieties of crops like wheat, peas, beans with high yields and high resistance to crop disease.
iv. Sterilization Medical instruments and equipments are sterilized by exposure to γ –rays. Gamma ray as are also being used to sterilize and preserve some food products. The method is safe as no radioactivity is induced in the material irradiated by γ- rays. Radiation has also been used to eliminate agricultural pests by sterilizing them and therefore serving the reproduction chains.
v. Carbon dating By measuring the residual activity of the quality remaining after death of an organism, we can determine how long ago the organism died. 2. Industrial uses (i) Tracers a) For investigation of flow of liquids in chemical plants or in underground water and sewerage pipelines. In the latter cases, a little radioactive solution is added to the liquid being pumped. Temporary high activity around a leak is detected from the ground above. The rate of flow of liquids can also be measured. b) For study of wear in machinery such as of piston rings in motor engine. Before the piston is put in place, it is irradiated with neutrons to form the radioisotope 59 Fe. As the piston rings wear out, the 59Fe which comes off with the oil is tested using Geiger Muller counter. Through comparison of the initial activity with the activity measured time, the rate of wear of the piston is deduced. c) Automatic control of thickness paper, plastic or metal sheeting as it gives through the production plant. The thickness is controlled by measuring the transmission of radiation through the sheet.
3. Diagnostic uses
Cobalt 60 and other γ- rays emitters are used as alternatives to X rays set ups which are more elaborate to produce radiographs for examination of welded beams and metal castings.
Nuclear fusion and fission Nuclear fission A nuclear fission reaction involves bombarding of the heavy nucleus with a highly energetic particles such as neutrons, protons, deuterons and alpha particles. The heavy nucleus splits into lighter nuclei of higher binding energy per nucleon. The mass deficiency which results is accounted for by the energy released in accordance to Einstein’s mass-energy relation. In most nuclear fission reactions, neutrons are used to induce a reaction because of being neutral, they can penetrate the nucleus. When 235U splits, it produces nuclei that are lighter and hence have higher binding energy. Examples of nuclear fission
In the above example, when the emitted neutrons encounter with other Uranium nuclides, they bombard the uranium and more splitting occurs with the release of more energy. The produced neutrons are called fission neutrons, and when this occurs, the reaction is called a chain reaction. In a chain reaction, a lot of energy is produced and unless this energy is controlled, the reactions may cause an explosion. Chain reaction is applied in making nuclear bombs. Nuclear fusion A lot of energy is released when the nuclei of lighter elements fuse together to form a heavy nucleus. The fusing together of nuclei to form a heavy nucleus is called nuclear fusion. Example Formation of alpha particles when lithium fuses with hydrogen.
The sun contains a considerable amount of hydrogen. It is believed that the energy of the sun is due to nuclear fusion of the hydrogen atoms. Fusion is capable if the nuclei concerned are able to approach each other close enough and if the temperatures are very high. These conditions are achieved in the sun.