Atomic Structure and the Periodic Table
Contents
2.1
The first chemist to use the name 'atom' was John Dalton (1766-1844). Dalton used the word 'atom' to mean the smallest particle of an element. He then went on to explain how atoms could react together to form molecules, he called 'compound atoms'.Therefore an Atom is smallest part of a element which can ever exist and a Molecule is the smallest part of a element or compound which can ever exist.
An outline of the discovery of cathode rays and the work of Rutherford on atomic structure
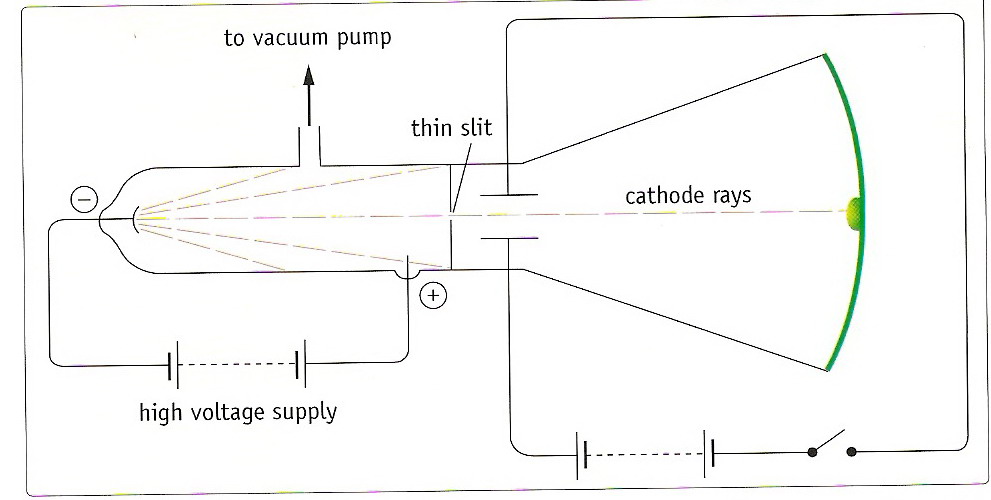
Firm evidence for the existence of electrons was not obtained until 1897. In that year, J.J Thomson was investigating the conduction of electricity by gases at very low pressure. At ordinary pressures gases are electrical insulators, but when they are subjected to very high voltages at very low pressures they 'break down' and conduct electricity. When the gas pressure is lowered to 0.001 atm and a potential difference of 5000 volts is applied across the tube containing the gas, the glass container begins to glow. When Thomson applied 15000 volts across the electrodes of a tube containing a trace of gas, a bright green glow appeared on the glass. The green glow results from the bombardment of the glass by rays travelling in straight lines from the cathode until they strike the anode or the glass walls of the tube. Thomson called these rays cathode rays.
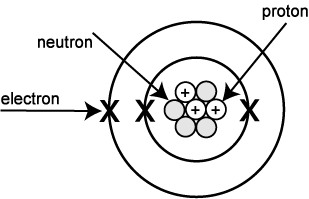
Atoms are made up of three types of subatomic particles called protons, neutrons and electrons.
The mass and charge of these subatomic particles is as follows:
SUB-ATOMIC PARTICLE MASS CHARGE Proton 1 +1 Neutron 1 0 Electron nearly 0 -1
The protons and neutrons are in the nucleus (centre) of the atom and the electrons orbit round the outside in shells (energy levels or layers). So you will often see pictures of atoms that look a little like this:
2.2 The Nucleus of the Atom
Isotopes and mass number
How many protons, neutrons and electrons does an atom have?
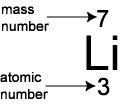
You can work this out using the periodic table. Every element in the periodic table has two numbers with it: the atomic number and the mass number. For example for lithium, the numbers are:
The atomic number is the number of protons that the atom has. It is also the number of electrons that the atom has. So lithium has 3 protons and 3 electrons.
The mass number is the number of protons and neutrons added together. So, for lithium there are 7 protons and neutrons combined, and we know that 3 of them are protons so there must be 4 neutrons.
RULE: The number is neutrons is (mass number - atomic number)
Atoms with the same atomic number, but different mass numbers are called Isotopes.
Stable and unstable isotopes: radioactivity. For example, the most common isotope of hydrogen has no neutrons at all; there's also a hydrogen isotope called deuterium, with one neutron, and another, tritium, with two neutrons.

Hydrogen Deuterium Tritium
Ordinary hydrogen is written 1H1, deuterium is 2H1, and tritium is 3H1
Light elements tend to have about as many neutrons as protons; heavy elements apparently need more neutrons than protons in order to stick together. Atoms with a few too many neutrons, or not quite enough, can sometimes exist for a while, but they're unstable. Unstable atoms are radioactive: their nuclei change or decay by spitting out radiation, in the form of particles or electromagnetic waves.
(a)The properties of alpha, Beta and gamma radiation and a brief treatment of the evidence for their existence.
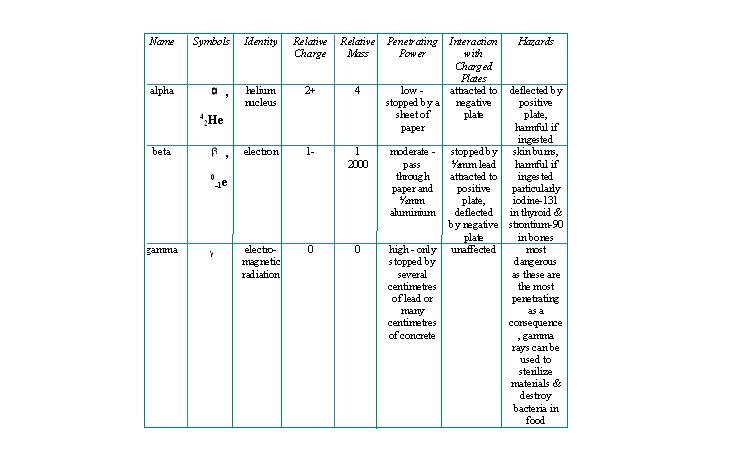
- The detailed physical evidence establishing the nature of alpha, Beta and gamma radiation is not required.
(b)Stability of radioactive nuclei as measured by their half-life.
At the beginning of the twentieth century detailed investigations were made of the disintegration of naturally occurring radioactive isotopes. Scientists were interested in:
1)the types of radioactive change (alpha-decay or beta-decay)
2)the products of decay (alpha-particles, beta-particles or gamma rays)
3)the rate of decay, and
4)the relatively stability of different radioactive isotopes.
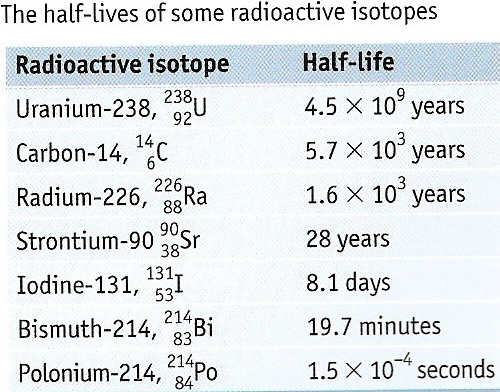
Experiments showed that the natural decay of a radioactive isotope could not be accelerated or retarded by physical or chemical means. for example, extremes of temperature and pressure had virtually no effect on the rate of decay. Therefore scientists found the most convenient method of expressing the rate of disintegration of a radioactive element was its half-life. The half- life of a radioactive isotope is the time taken for the amount or concentration of the isotope to fall to half of its original value.
(c) The changes in nuclei resulting from the loss of alpha, Beta and gamma rays: radioactive decay series.
(d)Uses of radioisotopes of importance in chemistry
1) Penetrating gamma-radiation from cobalt-60 is used in cancer treatment as tumour cells tend to be more susceptible to radiation than other cells.
2) Iodine-131 is used as Medical tracer to study & treat the thyroid gland & used in the diagnosis of adrenal medullary & for imaging suspected neural crest and other endocrine tumours
3) Iodine-123 is used in imaging to monitor thyroid function & detect adrenal dysfunction
4) Phosphorus-32 is used in the treatment of excess red blood cells
5)Magnesium-27 is used in the location of leaks in water pipes
6) Carbon-14 is used as a biological tracer, for example, in studies of photosynthesis
- A reader on "Uses of Radioactivity" will be prepared to illustrate this section. The students should be made aware of the usefulness of radioactivity.
2.3 The Electronic Structure of the Atom:
The atomic spectrum of hydrogen and other line spectra and successive ionization energies of elements as evidence for the existence of electronic energy levels.
Gaseous elements emit light when they are subjected to large potential differences in electric discharge in tubes. Neon advertising signs his way and the yellow sodium street lamps are discharge tubes containing sodium vapour. Thermal energy from the bunsen flame and electrical energy from the discharge tube have caused the elements to emit coloured light.
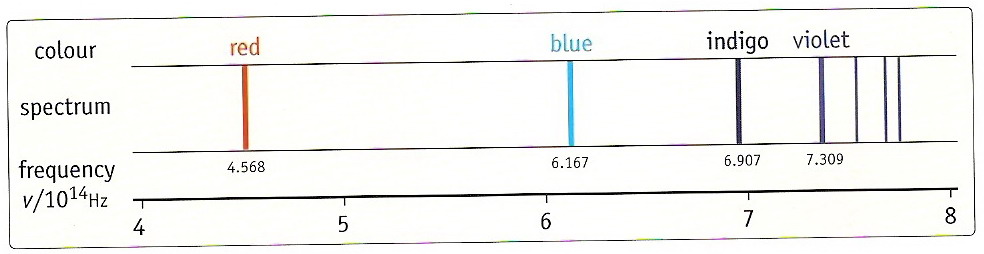
If the light emitted by these substances is examined using a spectroscope, it does not consist of a continuous range of colours like the spectrum of white light or the colours in a rainbow. Instead, the light emitted by these substances is composed of separate lines of different colour. This kind of spectrum is called a line emission spectrum.

Each line in an emission spectrum corresponds to light of a particular frequency.
The colours and frequencies of the more prominent lines in the visible region of the atomic hydrogen spectrum.
- A mathematical treatment of the hydrogen spectrum is not required. A graphical treatment to show the relationship between the convergence limit of the Lyman series and the ionization energy of hydrogen is recommended.
The relation between the line spectra of elements and electronic energy levels should by clearly brought out.
The spectral evidence for the existence of s, p and d sub-levels should be mentioned briefly.
The graph below shows the first ionisation energies of the first 20 elements plotted against atomic number. The graph can be divided into sections ending with a noble gas whose ionisation energy is higher than those of all the elements between it and the previous noble gas. The large ionisation energies of noble gases are another indication of their previous electronic structures and unreactivity.
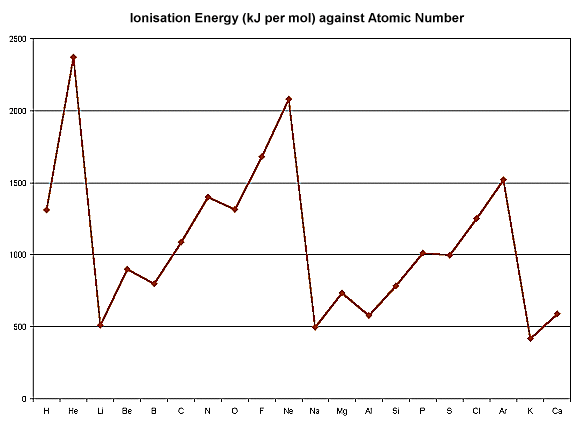
The points on the graph between one noble gas and the next correspond to the filling of one shell of electron. This means that the sub-sections of points correspond to sub-shells of electrons.
- The shapes of s, p and d orbitals are not required and will not be examined. The fact that they have slightly different energies is important and must be mentioned.
2.4 The Periodic Table
Atomic number as the basic classification of the elements in the Periodic table.
The Origin of the Periodic Table
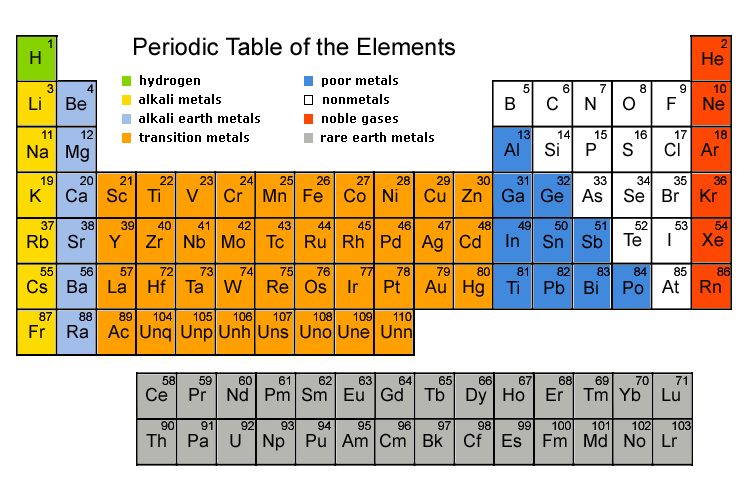
Early Chemists studied the group of known elements, and tried to come up with logical classification systems based on what they knew.An early attempt was made by a German Chemist named Johann Dobereiner, in 1817. Dobereiner noticed that there were several groups of three elements which shared certain properties. For example; chlorine, bromine and iodine all share antiseptic properties. Dobereiner called each group of three elements with similar properties a "triad". He also noticed that when he arranged the elements of a triad by atomic mass, the middle element had an atomic mass that fell close to the middle of the other two atomic masses. Almost fifty years later, an English Chemist named John Newlands proposed an updated classification system. In the 1860's a German Chemist named Lothar Meyer was developing a periodic table based on the idea that when the elements were arranged by atomic mass, certain properties were repeated periodically. There are two main classifications in the periodic table, "groups" and "periods." Groups are the vertical columns that include elements with similar chemical and physical properties. Periods are the horizontal rows.
- The rows (horizontal) of the periodic table are called PERIODS
- The columns (vertical) are called GROUPS
- Every group has a NUMBER
- Elements in the same group have SIMILAR CHEMICAL PROPERTIES
- This is because elements in the same group have the SAME NUMBER of electrons in their outer shell.
- For example, all of the elements in Group 1 (The alkali metals) - lithium, sodium, potassium etc. - have 1 ELECTRON IN THEIR OUTER SHELL.
Below is the periodic table:-
Group 1A
* Known as Alkali Metals * Very reactive * Never found free in nature * React readily with water
Group 2A
* Known as Alkaline earth elements * All are metals * Occur only in compounds * React with oxygen in the general formula EO (where O is oxygen and E is Group 2A element)
Group 3A
* Metalloids * Includes Aluminum (the most abundant metal in the earth) * Forms oxygen compounds with a X2O3 formula
Group 4A
* Includes metals and non-metals * Go from nonmetals at the top of the column to metals at the bottom * All oxygen form compounds with a XO2 formula
Group 5A
* All elements form an oxygen or sulfur compound with E2O3 or E2S3 formulas
Group 6A
* Includes oxygen, one of the most abundant elements. * Generally, oxygen compound formulas within this group are EO2 and EO3
Group 7A
* Elements combine violently with alkali metals to form salts * Called halogens, which mean "salt forming" * Are all highly reactive
Group 8A
* Least reactive group * All elements are gases * Not very abundant on earth * Given the name noble gas because they are not very reactive
- Questions will not be set on the historical development of the Periodic table. The work of Mosely in establishing the importance of atomic number should be mentioned.
Electronic structure as the basis for the periodicity in chemical and physical properties.
(a) The filling of electronic energy levels and pairing of electrons.
- The relative energies of the s, p and d levels and the number s, p and d orbitals should be brought in here.
(b) The periodicity of electronic structure, leading to the periodicity of chemical properties.
TEST YOURSELF WITH THE QUIZZES: